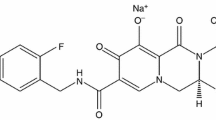
Abstract
Raltegravir (ISENTRESS®) is an HIV-1 integrase strand transfer inhibitor that is well established as a component of highly active antiretroviral therapy regimens for the treatment of adults with HIV-1 infection, and has recently been approved for the treatment of HIV-1-infected children and adolescents aged 2–18 years. A new chewable formulation has been introduced and results of a pharmacokinetic study have led to the establishment of dosages for this formulation for children. In a phase I/II, open-label, multicentre, clinical trial, raltegravir (administered as the chewable or the film-coated tablet) in combination with optimized background antiretroviral therapy was an effective treatment for treatment-experienced children and adolescents with HIV-1 infection, in terms of virologic measures of efficacy (i.e. a decrease of in HIV-1 RNA levels of ≥1 log10, or an HIV-1 RNA level of <400 copies/mL at the 24-week primary efficacy assessment), with virologic efficacy sustained at the 48-week assessment. Immunologic improvements (increases from baseline in CD4+ cell counts) were also observed. As a component of combination therapy, raltegravir was generally well tolerated over a period of up to 48 weeks. Raltegravir is an important new option for the treatment of children and adolescents with HIV-1 infection, and the introduction of a new chewable formulation (allowing dosage flexibility) extends its benefits to the treatment of younger children.

Similar content being viewed by others
References
United Nations. UNAIDS World AIDS Day Report 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf. Accessed 6 Nov 2013.
UNICEF. Unite for children. Unite against AIDS. http://www.unicef.org/aids/index_preventionyoung.html. Accessed 6 Nov 2013.
Eley BS, Meyers T. Antiretroviral therapy for children in resource-limited settings: current regimens and the role of newer agents. Pediatric Drugs. 2011;13:303–16.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 6 Nov 2013.
Fraaij PLA, Van Kampen JJA, Burger DM. Pharmacokinetics of antiretroviral therapy in HIV-1-infected children. Clin Pharmacokinet. 2005;44:935–56.
United Nations. UNAIDS strategy 2011–2015. http://www.unaids.org/en/aboutunaids/unaidsstrategygoalsby2015/. Accessed 6 Nov 2013.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013. http://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed 6 Nov 2013.
HHS Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2012. http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed 6 Nov 2013.
PENTA Steering Committee. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613.
ClinicalTrials.gov. Safety and effectiveness of raltegravir in HIV-infected children and adolescents. 2013. http://clinicaltrials.gov/show/NCT00485264. Accessed 29 Oct 2013.
Merck and Co Inc. FDA Approves Merck’s ISENTRESS® (raltegravir) for use in children ages two years and older as part of HIV-1 combination therapy. 2012. http://www.mercknewsroom.com/press-release/prescription-medicine-news/fda-approves-mercks-isentress-raltegravir-use-children-ages. Accessed 29 Oct 2013.
i-base HIV treatment bulletin. EU approval for raltegravir in children aged 2 years and older. 2013. http://i-base.info/htb/21350. Accessed 6 Nov 2013.
European Medicines Agency. Isentress (raltegravir): summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000860/WC500037405.pdf. Accessed 29 Oct 2013.
Merck and Co Inc. US prescribing Information for ISENTRESS® (raltegravir). 2013. http://www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi.pdf. Accessed 29 Oct 2013.
Merck and Co. Inc. FDA approves new U.S. labeling for ISENTRESS® (raltegravir) to include 240-week results from STARTMRK study of ISENTRESS containing regimen in previously untreated HIV-1 Infected adult patients http://www.businesswire.com/news/home/20130701006520/en/FDA-Approves-U.S.-Labeling-ISENTRESS%C2%AE-raltegravir-Include. Accessed 6 Nov 2013.
Croxtall JD, Lyseng-Williamson KA, Perry CM. Raltegravir. Drugs. 2008;68(1):131–8.
Croxtall JD, Keam SJ. Raltegravir: a review of its use in the management of HIV infection in treatment-experienced patients. Drugs. 2009;69(8):1059–75.
Croxtall JD, Scott LJ. Raltegravir: in treatment-naive patients with HIV-1 infection. Drugs. 2010;70(5):631–42.
Nachman S, Acosta E, Zheng N, et al. IMPAACT P1066: raltegravir (RAL) safety and efficacy in HIV infected (+) youth 2 to 18 years of age through week 48 [slide presentation]. XIX International AIDS Conference; 2012 Jul 22–27; Washington, DC.
Nachman S, Zheng N, Acosta E, et al. Pharmacokinetics, safety and 48 week efficacy of oral raltegravir in Human Immunodeficiency Virus type-1 (HIV) infected children 2 through 18 years of age. Clin Infect Dis. 2013 Oct 21. doi:10.1093/cid/cit696.
International Maternal Pediatric Adolescent AIDS Clinical Trials Group IMPAACT. IMPAACT Enrolling Studies. https://impaactgroup.org/impaact-enrolling-studies. Accessed 6 Nov 2013.
Brainard D, Gendrano III I, Jin B, et al. Comparison of adult and pediatric formulations of raltegravir (RAL) in healthy adults [abstract no. S-197]. 17th Conference on Retroviruses and Opportunistic Infections; 2010 February 16–19; San Francisco CA. USA.
Brainard DM, Wenning LA, Stone JA, et al. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J Clin Pharmacol. 2011;51:1376–402.
Nachman S, Pacosta E, Wiznia A, et al. Raltegravir pharmacokinetics and safety in adolescents: preliminary results from IMPAACT P1066 [abstract/poster no. H-4059a]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America—A Joint Meeting: Late Breaker Abstracts; 2008 Oct 25–28, Washington, DC, USA.
Fitzgerald F, Penazzato M, Gibb D. Development of antiretroviral resistance in children with HIV in low- and middle-income countries. J Infect Dis. 2013;207:S85–92.
Nachman S, Acosta E, Zheng N, et al. (Issentress) raltegravir for pediatrics: interim results from IMPAACT P1066: raltegravir (RAL) oral chewable tablet (CT) formulation in children 2–5 years [abstract]. 18th Conference on Retroviruses and Opportunistic Infections; 2011 Feb 27–Mar 2; Boston, MA, USA.
Spector SA, Acosta E, Zheng N, et al. Interim results from IMPAACT P1066: raltegravir oral granule formulation in children 6 months to <2 years [abstract no. S1001/poster no. 987]. 19th Conference on Retroviruses and Opportunistic Infections; 2012 Mar 3-6; Seattle, WA, USA.
Frenkel L, Nachman S, Zheng L, et al. Interim results from IMPAACT P1066: dose-finding pharmacokinetics and 24- and 48-week safety and efficacy of raltegravir granules for suspension formulation in human immunodeficiency virus type-1-infected infants starting treatment at 4 weeks–6 months of age [abstract and poster no. 42265]. ID Week; 2013 Oct 2–6 San Francisco, CA, USA.
Yilmaz A, Gisslen M, Spudich S, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS ONE. 2009;4(9):e6877.
Kassahun K, McIntosh I, Cui D, et al. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos. 2007;35(9):1657–63.
University of Liverpool. HIV drug interactions. http://www.hiv-druginteractions.org/. Accessed 6 Nov 2013.
Nachman S, Frenkel LM, Samson P, et al. 24 week safety and efficacy from IMPAACT P1066: a phase I/II, multicenter, open-label, noncomparative study to evaluate raltegravir in HIV-1 infected youth [abstract no. H-924a]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco, CA, USA.
Nachman S, Acosta E, Samson P, et al. Interim results from IMPAACT P1066: Raltegravir oral chewable tablet formulation in children 6 to 11 years [abstract 161LB]. 17th Conference on Retroviruses and Opportunistic Infections; 2010 Feb 27–Mar 2, San Francisco, CA, USA.
Nachman S, Acosta E, Zheng N, et al. Interim results from IMPAACT P1066: RAL oral chewable tablet formulation for 2- to 5-year-olds [abstract no. 715]. 18th Conference on Retroviruses and Opportunistic Infections; 2011 Feb 27–Mar 2; Boston, MA, USA.
Wiznia A, Samson P, Acosta E, et al. Safety and efficacy of raltegravir in pediatric HIV infection. Preliminary analysis from the International Maternal Pediatric Adolescent AIDS Clinical Trials Group, P1066 [abstract no. 874]. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8–11; Montreal, CA, USA.
Nachman S, Samson P, Acosta E, et al. Pharmacokinetic, safety, and efficacy data on cohort IIA; youth aged 6 to 11 years from IMPAACT P1066: A phase I/II study to evaluate raltegravir in HIV-1 infected youth [poster 873]. 17th Conference on Retroviruses and Opportunistic Infections; 2010 Feb 16–19; San Francisco, CA, USA.
Thuret I, Tamalet C, Reliquet V, et al. Raltegravir in children and adolescents: The French Expanded Access Program [abstract no. 873]. 16th Conference on Retroviruses and Opportunistic Infections; 2009 Feb 8–11; Montreal, Canada.
Thuret I, Chaix M-L, Tamalet C, et al. Raltegravir, etravirine and r-darunavir combination in adolescents with multidrug-resistant virus. AIDS. 2009;23(17):2364–6.
Briz V, Leon-Leal JA, Palladino C, et al. Potent and sustained antiviral response of raltegravir-based highly active antiretroviral therapy in HIV type 1-infected children and adolescents. Pediatr Infect Dis J. 2012;31(3):273–7.
Merck & Co. Inc. Pediatric Treatments for HIV http://www.merckresponsibility.com/focus-areas/access-to-health/committed-to-improving-access-to-hiv-care/hiv-research/pediatric-treatments-for-hiv/. Accessed 29 Oct 2013.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: B. S. Eley, Paediatric Infectious Diseases Unit, Red Cross War Memorial Children’s Hospital, University of Cape Town, Cape Town, South Africa; V. Giacomet, Infectious Disease Unit, Department of Paediatrics, Luigi Sacco Hospital, Milan, Italy; S. Nachman, Department of Pediatrics, State University of New York at Stony Brook, Stony Brook, New York, USA; J. T. Ramos, Infectious Disease Unit, Hospital Universitario de Getafe, Getafe, Madrid, Spain.
Rights and permissions
About this article
Cite this article
Perry, C.M. Raltegravir: A Review of Its Use in the Management of HIV-1 Infection in Children and Adolescents. Pediatr Drugs 16, 91–100 (2014). https://doi.org/10.1007/s40272-013-0058-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-013-0058-9