Abstract
Background and Objectives
This study was conducted to investigate the effect of high-fat meals on the pharmacokinetics (PK) and safety profile of SAF-189s, a novel ALK/ROS1 inhibitor.
Methods
This was a single-center, phase I, open-label, crossover study in which healthy adults (≥18 years) were randomized (1:1) to two sequences of SAF-189s administration (fasted-fed or fed-fasted) separated by a 14-day washout. After a ≥10-h overnight fast, volunteers received SAF-189s 160 mg orally in a fasted state or 30 min after a high-fat, high-calorie meal. Similarity of pharmacokinetic parameters was concluded if the 90% CI for the geometric mean ratio (GMR) between the fed and fasted group fell within the predefined range of 0.80–1.25.
Results
In total, 24 subjects were enrolled and 23 completed the study. SAF-189s maximum plasma concentration (Cmax; GMR: 109.1% [90% CI 103.1–115.4]) was comparable under fed (high-fat meal, n = 24) versus fasted (n = 23) conditions, with no effect on area under the plasma concentration–time curve from time 0 to t (AUC0-t; GMR: 105.1% [90% CI 100.3–110.2]) and AUC from time 0 to infinity (AUC0-∞; GMR: 105.5% [90% CI, 100.6–110.6]). In both groups, the median time to maximum plasma concentration (tmax) was around 6 h and mean plasma half-life (t½) was around 35 h. Fed administration led to a lower incidence of treatment-emergent adverse events (TEAEs; 29.2% vs 54.2%), including gastrointestinal disorders (4.2% vs 41.7%) and headache (0.0% vs 12.5%), versus fasted administration.
Conclusions
A high-fat meal had minimal effect on the pharmacokinetic profile of SAF-189s compared with a fasted state following a single dose of 160 mg. Administration with a high-fat meal led to a lower incidence of TEAEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High-fat food had minimal effect on the pharmacokinetic profile of SAF-189s compared with administration in the fasted state, following a single dose of 160 mg. |
SAF-189s had a manageable safety profile and administration with a high-fat meal led to a lower incidence of treatment-emergent adverse events. |
1 Introduction
Lung cancer is the leading cause of cancer-related death worldwide and the second-most common cancer by prevalence in both men and women, accounting for approximately 18% of all deaths from cancer in 2020 [1]. In China, lung cancer is both the most common cancer type and the leading cause of cancer mortality [2]. Non-small cell lung cancer (NSCLC) is the most common category of lung cancer globally and in China, accounting for over 80% of lung cancer cases [3]. For patients with advanced NSCLC, molecular targeted therapies have become a key part of standard treatment. This is reflected in current NSCLC treatment guidelines, which recommend molecular testing at diagnosis for sensitizing genetic variants including epidermal growth factor receptor (EGFR) mutations, BRAF mutations, METex14 skipping mutations, RET rearrangements, anaplastic lymphoma kinase (ALK) fusions and re-arrangements/fusions of the oncogenic c-ros oncogene (ROS1) [4, 5]. For patients with NSCLC harboring sensitizing genetic variants, targeted therapy offers superior outcomes and lower toxicity compared with traditional chemotherapy [5].
ALK and ROS1 are well known mutated/re-arranged oncogenic genes observed in patients with NSCLC; ALK re-arrangements are present in 2–8% [6, 7] and ROS1 re-arrangements in 1–2% of this patient population. Furthermore, ROS1 and ALK re-arrangements are usually mutually exclusive of each other and of re-arrangements or mutations in EGFR, KRAS, BRAF and METex14 [8,9,10,11,12,13]. EGFR wild-type NSCLC is associated with a higher prevalence of ALK and ROS1 re-arrangements, as high as 12.2% and 4.4%, respectively [14, 15]. Patients with advanced or metastatic NSCLC harboring ALK or ROS1 gene re-arrangements (hereafter referred to as ALK- or ROS1-positive NSCLC) are highly sensitive to targeted therapy with tyrosine kinase inhibitors (TKIs) [16, 17].
SAF-189s is an ALK/ROS1 inhibitor designed to provide effective CNS penetration and optimized pharmacokinetic (PK) and pharmacodynamic properties. Preclinical data have shown sub-nanomolar to nanomolar IC50 (half maximal inhibitory concentration) values for SAF-189s against ALK and wild-type and mutant ROS1 kinase activity [18]. This included potent inhibition of cell proliferation in HCC78 and BaF3 cells expressing ROS1 fusion wild-type and resistance mutants [18]. SAF-189s also inhibited growth of ROS1 wild-type and G2032R mutant xenograft models [18]. SAF-189s is mainly metabolized by cytochrome P450 3A4 (CYP3A4) in human liver microsomes. Because of the potential for drug–drug interactions, concomitant use of SAF-189s with strong CYP3A inhibitors and inducers should be avoided. PK data for single doses of SAF-189s in the range of 20–210 mg in patients with ALK-positive solid tumors showed that drug exposure increased with dose increase [19]. Preliminary efficacy and safety results from a phase I/II study, including 45 patients in phase I and 150 patients in phase II with ALK-positive NSCLC who had failed prior systemic therapy, showed that oral SAF-189s (20–210 mg/day) received in a fasted state was well tolerated, exerted promising anti-tumor efficacy and had intracranial activity. In phase I, an objective response rate (ORR) of 62.2% (28/45; 95% confidence interval [CI] 46.5–76.2) was reported, with ORRs of 72.7% (8/11; 95% CI 39.0–94.0) and 63.0% (17/27; 95% CI 42.4–80.6) in ALK inhibitor-naive and brain metastases subgroups, respectively. In phase II, the IRC-assessed ORR was 77.3% (116/150; 95% CI 69.8–83.8) with an ORR of 91.3% (95/104; 95% CI 84.2–96.0) in ALK inhibitor-naive patients; 65.4% (17/26; 95% CI 44.3–82.8) in the crizotinib-pretreated group and 70.4% (50/71; 95% CI 58.4–80.7) in the brain metastases subgroup [20].
Here, we report results of a phase I study conducted to evaluate the effect of high-fat food on the PK profile and safety of SAF-189s 160 mg in healthy Chinese adults. The 160-mg dose was selected based on data from the phase I/II study, which identified 160 mg as the recommended phase II dose and found that single doses of SAF-189s 160 mg are generally well tolerated, with most adverse events (AEs) categorized as Grade 1 or 2 [20].
2 Materials and Methods
2.1 Study Design and Inclusion Criteria
The protocol for this study was approved by the Ethics Review Board at The Second Hospital of Anhui Medical University (approval number: YW2021-127). The study was registered at the Chinese Clinical Trial Registry (www.chictr.org.cn; ChiCTR2200067008, 23 December 2022). All subjects provided written, informed consent before entering this single center, randomized sequence, open-label, crossover food effect study conducted at The Second Hospital of Anhui Medical University, China. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and subsequent revisions, and Chinese Guidelines for Good Clinical Practice.
The study included healthy Chinese adults (aged ≥ 18 to ≤ 45 years) with a body weight of ≥ 45 kg for females and ≥ 50 kg for males, body mass index (BMI) of ≥ 19.0 to ≤ 26.0 kg/m2 and no clinically significant abnormalities. All subjects were required to agree to take appropriate and effective contraceptive measures from 2 weeks before signing informed consent until 6 months after the last administration of SAF-189s.
Key exclusion criteria included any history of gastrointestinal diseases affecting drug absorption, distribution, metabolism or excretion, positivity for HBV surface antigen, HCV antibodies, treponema pallidum antibodies and HIV antibodies, receiving any drug or Chinese herbal medicine < 14 days before screening, or receiving any drug that affects liver metabolic enzymes < 28 days before screening, smoking > 5 cigarettes/day < 3 months before screening, history of alcoholism (defined as > 14 units per week) < 3 months before screening, consumption of foods that may affect drug absorption and metabolism < 48 h before study drug administration (including pitaya, mango, grapefruit, chocolate, high xanthine foods, or drinks containing caffeine and alcohol) and pregnant or lactating women.
2.2 Participant Allocation and Study Drug Administration
Eligible volunteers were randomized (1:1) to one of two sequences of drug administration (fasted-fed or fed-fasted) separated by a 14-day washout period. After a ≥10-h overnight fast, volunteers received SAF-189s 160 mg (4× 40 mg capsules; Fochon Pharmaceuticals Ltd) in a fasted state, or 30 min (± 1 min) after starting to eat a high-fat, high-calorie meal (approximately 56% of calories from fat, 17% of calories from protein, 27% of calories from carbohydrates and a total of 890 kcal), with 240 mL of warm water. All volunteers were forbidden from drinking from 1 h before until 2 h after receiving SAF-189s and were required to fast for 4 h after administration. Randomization was implemented with a random number table generated in SAS v9.4 (SAS Institute, USA) and using block randomization.
2.3 Pharmacokinetic Sample Collection and Analysis
PK blood samples of approximately 3 mL were collected after each SAF-189s administration at pre-dose and at 1, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 120 and 168 h post-dose into vacuum blood collection tubes, and gently inverted several times to ensure mixing with the anticoagulant. Each sample was centrifuged at 1700g at 2–8 °C for 10 min and the separated plasma aliquots were stored at −70 °C until analysis. Plasma concentrations of SAF-189s were measured at a central laboratory using validated high performance liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The LC-MS/MS system was the Triple Quad™ 5500 (SCIEX), coupled with the LC-30AD series high-performance liquid chromatography (Shimadzu Corporation). The analytical column was carried out using a Polaris C18-A (50 × 3.0 mm; VWR international) with a mobile phase consisting of (A) water with formic acid 0.1%/ammonium formate 2 mM; (B) 0.1% formic acid in acetonitrile/water (95:5, v:v)/2 mM ammonium acetate. SAF-189s was analyzed by electron spray ionization in positive ion mode, using a monitoring ratio of 570.3 to 98.3 for SAF-189s. The standard curve range was 0.5–150 ng/mL for SAF-189s. The assay accuracy was between −3.5% and 1.5% (−4.5% to 1.1% for lower limit of quantification [LLOQ]), and the maximum within-day and between-day precision (coefficient of variation) was 3.3% (4.0% for LLOQ).
The following PK parameters were calculated using a non-compartmental analysis method in Phoenix WinNonlin v8.3 (Certara, USA): the area under the concentration–time curve from 0 to last measurable concentration, and 0 to infinity (AUC0-t, AUC0-∞), and the maximum plasma concentration (Cmax), tmax, terminal elimination half-life (t½) and apparent oral clearance (CL/F). Each sampling time point was taken as the actual sampling time, and plasma drug concentrations below the LLOQ were treated as 0 before Cmax was reached or as ‘missing’ after Cmax. For all descriptive analyses, values below the LLOQ were recorded as 0. In the calculation of plasma concentration, missing data were recorded as ‘missing’ and concentrations below the LLOQ were recorded as ‘below quantifiable limit’.
2.4 Safety Assessment
Safety evaluations included measurements of vital signs, electrocardiograms (ECGs), routine clinical laboratory analyses and recording of AEs. AEs were reported and graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 and coded using the ICH international Medical Dictionary for Regulatory Activities (MedDRA) version 24.1 and summarized according to system organ classification (SOC) and preferred term (PT). A treatment-emergent AE (TEAE) was defined as any AE not present prior to the initiation of SAF-189s or any event already present that worsened in intensity or frequency following SAF-189s administration.
2.5 Statistical Methods
Based on previous phase I PK data, the sample size was calculated by assuming a geometric mean ratio of 1.05, and an intra-subject coefficient of variation (CV) of 20%. Based on the above assumptions, 18 evaluable patients were required to obtain 80% power to observe the 90% CI of the geometric mean ratio within the pre-specified equivalence limits of 0.80–1.25. Accounting for a dropout rate of 20%, the total enrollment target was therefore set at 24 subjects (12 per dosing sequence group).
Cmax and AUC were calculated for each group to determine whether the high-fat, high-calorie meal had a significant effect on the PK of SAF-189s. The relative bioavailability following fasted and fed conditions was evaluated by converting Cmax, AUC0-t and AUC0-∞ to a natural logarithm for a linear mixed model. The linear mixed model included sequence, period and treatment (fasted/fed) as fixed effects, and the subjects nested in sequence as a random effect. The food effect was evaluated using the estimated least-squares geometric mean ratios of Cmax, AUC0-t and AUC0-∞ for fed and fasted conditions and their corresponding 90% CI. If the 90% CIs of the geometric mean ratio for Cmax and AUC were completely contained in the equivalence limits of 0.80–1.25, taking the fasted group as reference, then it could be concluded that high-fat meals had no significant effect on the PK parameters of the SAF-189s capsule.
SAF-189s plasma concentration data were calculated in the pharmacokinetics concentration set (PKCS), defined as all randomized subjects who received at least one dose of study drug and had at least one valid measurement of plasma drug concentration. PK parameters were calculated in the pharmacokinetics parameter set (PKPS), defined as all randomized subjects who received at least one dose of study drug. The equivalence of SAF-189s absorption degree and speed under fasted and fed conditions was evaluated in the bioavailability analysis set (BAS), defined as all subjects with at least one evaluable PK parameter. Safety was evaluated in all subjects who received at least one dose of study drug. All statistical analyses were performed using SAS v9.4.
3 Results
3.1 Demographics
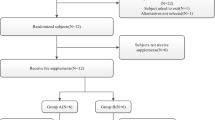
A total of 24 healthy Chinese volunteers underwent randomization; 12 to each SAF-189s administration sequence (Fig. S1, see electronic supplementary material [ESM]). One volunteer randomized to the fed-fasted group did not complete the study due to an AE (Grade 1 vomiting) and was excluded from the PKPS for fasted administration of SAF-189s as only one blood sample was collected before withdrawal. All other volunteers completed the study. Volunteer demographics and baseline characteristics were well balanced across the two administration sequence groups (Table S1, see ESM).
3.2 Pharmacokinetics
The mean concentration−time curves for SAF-189s 160 mg administered orally in a fasted state and after a high-fat meal are shown in Fig. 1. The PK parameters for SAF-189s 160 mg administered orally in a fasted state and after a high-fat meal are summarized in Table 1. Compared with administration in a fasted state, administration after a high-fat meal resulted in similar Cmax (geometric mean: 41.8 vs 37.8 ng/mL), AUC0-t (geometric mean: 1610 vs 1510 ng·h/mL) and AUC0-∞ (geometric mean: 1690 vs 1580 ng·h/mL) of SAF-189s. Inter-subject variability appeared similar under fasted and fed conditions for Cmax (23.0 vs 19.7%), AUC0-t (33.8 vs 31.3%) and AUC0-∞ (34.6 vs 32.8%). In both groups, the median tmax was around 6 h and the mean t½ was around 35 h.
3.3 Food Effect on Bioavailability of SAF-189s
High-fat meals had minimal effect on the PK profile of oral SAF-189s 160 mg. The geometric mean ratios (90% CI) of Cmax, AUC0-t and AUC0-∞ for the fed versus fasted administration groups were 109.1% (103.1–115.4), 105.1% (100.3–110.2) and 105.5% (100.6–110.6), respectively. The 90% CIs for Cmax, AUC0-t and AUC0-∞ were completely contained within the range of 0.80–1.25, a default bioequivalent boundary. The results indicated that high-fat meals had a minimal effect on the PK of SAF-189s (Table 2).
3.4 Safety
The incidence of TEAEs was lower when SAF-189s was administered after a high-fat meal compared with a fasted state (29.2% vs 54.2%) (Table 3). The incidence of gastrointestinal disorders was notably lower following fed administration of SAF-189s versus fasted administration (4.2% vs 41.7%) and the most common TEAEs in this category were nausea and abdominal pain. Headache was also less common following fed versus fasted administration of SAF-189s (0.0% vs 12.5%). All reported TEAEs were Grade 1 except for one volunteer with Grade 2 mouth ulceration, blood triglycerides increased and neutrophil count decreased following fasted administration and one volunteer with blood triglycerides increased after fed administration. No volunteer experienced a Grade ≥3 TEAE and no serious AEs were reported. Only one volunteer discontinued the study due to an AE (Grade 1 vomiting).
4 Discussion
In this phase I food effect trial, high-fat food had a minimal effect on the PK profile of SAF-189s compared with administration in a fasted state following a single dose, as indicated by the 90% CIs of the geometric mean ratios of Cmax, AUC0-t and AUC0-∞ for fed versus fasted administration, which fell within the pre-defined range (0.80–1.25). Despite this, administration with a high-fat meal led to a slight increase in median Cmax and AUC, while the mean tmax and t½ were unaffected. Administration with a high-fat meal appeared to improve the safety profile of SAF-189s, although safety was manageable following both fed and fasted administration. SAF-189s is a biopharmaceutics classification system (BCS) class IV drug [21] with low solubility and low gastrointestinal permeability. In vitro data show that SAF-189s is slightly soluble in 0.1 mol/L hydrochloric acid and has poor solubility at pH 6.8. Therefore, a high-fat meal could potentially elevate gastric pH and decrease SAF-189s solubility [22, 23]. However, a high-fat meal may also stimulate increased blood flow to the intestinal mucosa, which would facilitate the uptake of drug into the circulation [24] or enhance the dissolution rate of the drug by incorporation into micelles formed by bile salts [25, 26]. In these cases, an increase in SAF-189s bioavailability in the presence of a high-fat meal would be expected. In a Caco-2 permeability assay, SAF-189s showed low permeability at concentrations ranging from 2.00 to 50.0 µM, with evidence of drug efflux. A study by Wu and Benet noted that high-fat meals may inhibit efflux drug transporters [27], which may result in an increase in SAF-189s bioavailability. Therefore, our finding that a high-fat meal had no effect on the PK of oral SAF-189s is likely due to a combination of the above-mentioned effects.
A phase I trial of SAF-189s in patients with ALK-positive solid tumors reported PK data for nine patients following a single 160 mg dose of SAF-189s administered in fasted conditions. The findings were broadly comparable with the results of the present study when inter-patient variability is taken into account, with a geometric mean (CV%) Cmax of 41.9 (27.0) ng/mL (healthy volunteers: 41.8 ng/mL), AUC0-∞ of 1970 (30.0) ng·h/mL (healthy volunteers: 1690 ng·h/mL) and t½ of 39.3 (25.6) h (healthy volunteers: 35 h) [19]. A shorter median tmax was observed in patients compared with healthy volunteers (3.0 vs 6.0 h), which is likely related to the variability and different PK sampling schedule in patients.
In our study, exposure to oral SAF-189s was similar following administration in fasted or fed states. This finding is consistent with food effect studies showing no clinically meaningful effect of food on drug exposure for oral crizotinib [28], the second-generation oral ALK inhibitor brigatinib [29], the third-generation agent lorlatinib [30] and the ALK/ROS1 inhibitor entrectinib [31]. In contrast, food effect studies of the second-generation ALK TKIs alectinib [32, 33] and ceritinib [34] showed a positive effect of administration with food, and the labelling for these drugs therefore recommends administration with food. Interestingly, although SAF-189s was developed based on ceritinib, it appears to have a differential food effect. Finally, differing from the other ALK or ROS1 inhibitors with published food effect results, the ALK TKI ensartinib showed reduced exposure following administration with food compared with fasted administration [35].
Our results show that single doses of SAF-189s 160 mg were generally well tolerated, but the safety profile was moderately improved with administration following a high-fat meal versus fasted administration, with an overall incidence of TEAEs of 29.2% vs 54.2%, respectively. In particular, the incidence of gastrointestinal disorders and headache were higher following fasted administration of SAF-189s. However, it should be noted that the vast majority of TEAEs were Grade 1 and only one patient discontinued the study due to an AE (Grade 1 vomiting). Differential effects of administration with food on safety and tolerability have also been reported for the currently approved ALK and ROS1 TKIs with different BCS classifications. For example, no effect of food on PK and safety profiles was observed for crizotinib (BCS class IV) [28], brigatinib (BCS class I) [29], lorlatinib (BCS class IV) [30] or entrectinib (BCS class II) [31], and their respective labelling indicates administration with or without food. Conversely, compared with fasted administration, alectinib (BCS class IV) taken with food showed improved exposure with no effect on safety [32, 33] and ceritinib (BCS class IV) showed improved exposure and safety with food [34]. Both alectinib and ceritinib suggest administration with food in their labels. Since it is difficult to accurately predict both the direction and magnitude of food effect for class IV compounds, a food effect study would be needed to study the effect of food on the PK of BCS class IV drugs to identify the optimal dosing approach.
This study had several potential limitations. Firstly, the study included only Chinese participants, which may limit the generalizability of the findings to other populations. Similarly, the single-center study design may also limit the generalizability of the results. Secondly, the study was conducted in healthy volunteers, and therefore did not account for the effect of comorbidities and concomitant cancer medications on the PK and safety of SAF-189s.
5 Conclusion
In healthy Chinese volunteers, the PK of a single oral dose of SAF-189s 160 mg was similar following fed and fasted administration and the safety profile was manageable, with a lower incidence of TEAEs reported following fed administration. SAF-189s is currently under investigation in patients with advanced ALK-or ROS1-positive NSCLC in a multicenter phase II trial (ClinicalTrials.gov identifier: NCT04237805).
Availability of Data and Materials
The data underlying this study are not publicly available as they include patient-level data, but are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–91. https://doi.org/10.1097/cm9.0000000000001474.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. https://doi.org/10.4065/83.5.584.
National Comprehensive Cancer Network. Non-small cell lung cancer. (Version 1.2022). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed February 25, 2022.
Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–9. https://doi.org/10.1016/j.semcancer.2017.11.019.
Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, Shyr Y, Camidge DR, Sequist LV, Glisson BS, Khuri FR, Garon EB, Pao W, Rudin C, Schiller J, Haura EB, Socinski M, Shirai K, Chen H, Giaccone G, Ladanyi M, Kugler K, Minna JD, Bunn PA. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. https://doi.org/10.1001/jama.2014.3741.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. https://doi.org/10.1038/nature05945.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70. https://doi.org/10.1200/jco.2011.35.6345.
Kim HR, Lim SM, Kim HJ, Hwang SK, Park JK, Shin E, Bae MK, Ou SH, Wang J, Jewell SS, Kang DR, Soo RA, Haack H, Kim JH, Shim HS, Cho BC. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol. 2013;24(9):2364–70. https://doi.org/10.1093/annonc/mdt220.
Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19(15):4040–5. https://doi.org/10.1158/1078-0432.Ccr-12-2851.
Alì G, Proietti A, Pelliccioni S, Niccoli C, Lupi C, Sensi E, Giannini R, Borrelli N, Menghi M, Chella A, Ribechini A, Cappuzzo F, Melfi F, Lucchi M, Mussi A, Fontanini G. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449–58. https://doi.org/10.5858/arpa.2013-0388-OA.
Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ, Iafrate AJ, Arcila ME, Ladanyi M, Engelman JA, Dias-Santagata D, Shaw AT. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–81. https://doi.org/10.1158/1078-0432.Ccr-13-0318.
Takahashi T, Sonobe M, Kobayashi M, Yoshizawa A, Menju T, Nakayama E, Mino N, Iwakiri S, Sato K, Miyahara R, Okubo K, Manabe T, Date H. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17(3):889–97. https://doi.org/10.1245/s10434-009-0808-7.
Xu Y, Chang H, Wu L, Zhang X, Zhang L, Zhang J, Li Y, Shen L, Zhu X, Zhou X, Bai Q. High prevalence of ROS1 gene rearrangement detected by FISH in EGFR and ALK negative lung adenocarcinoma. Exp Mol Pathol. 2020;117: 104548. https://doi.org/10.1016/j.yexmp.2020.104548.
Song Z, Zheng Y, Wang X, Su H, Zhang Y, Song Y. ALK and ROS1 rearrangements, coexistence and treatment in epidermal growth factor receptor-wild type lung adenocarcinoma: a multicenter study of 732 cases. J Thorac Dis. 2017;9(10):3919–26. https://doi.org/10.21037/jtd.2017.09.79.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. https://doi.org/10.1056/NEJMoa1006448.
Patil T, Simons E, Mushtaq R, Pacheco JM, Doebele RC, Bowles DW. Targeted therapies for ROS1-rearranged non-small cell lung cancer. Drugs Today (Barc). 2019;55(10):641–52. https://doi.org/10.1358/dot.2019.55.10.3030646.
Xia ZJ, Ji YC, Sun DQ, Peng X, Gao YL, Fang YF, Zhao XD, Wang WB, Ding J, Geng MY, Ai J. SAF-189s, a potent new-generation ROS1 inhibitor, is active against crizotinib-resistant ROS1 mutant-driven tumors. Acta Pharmacol Sin. 2021;42(6):998–1004. https://doi.org/10.1038/s41401-020-00513-3.
Yang J, Zhou J, Cheng Y, Li M, Zhao Q, Zhang Z, Zang A, Fan Y, Hui AM, Zhou Y, Wu Z, Sun J, Pan Z, Qiu J, Wu YL. SAF-189s in advanced, ALK-positive, non-small cell lung cancer: results from a first-in-human phase 1/2, multicenter study. Abstract 9076. J Clin Oncol 40, 2022 (suppl 16; abstr 9076). https://doi.org/10.1200/JCO20224016_suppl9076
Yang J-J, Zhou J, Yang N, Wu Z, Sun J, Hui A-M, Wu Y-L. SAF-189s in previously treated patients with advanced ALK-rearranged non-small cell lung cancer (NSCLC): results from the dose-finding portion in a single-arm, first-in-human phase I/II study. J Clin Oncol. 2020;38(15_suppl): e21689. https://doi.org/10.1200/JCO.2020.38.15_suppl.e21689.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. https://doi.org/10.1023/a:1016212804288.
Abuhelwa AY, Williams DB, Upton RN, Foster DJ. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm. 2017;112:234–48. https://doi.org/10.1016/j.ejpb.2016.11.034.
Veerman GDM, Hussaarts K, Jansman FGA, Koolen SWL, van Leeuwen RWF, Mathijssen RHJ. Clinical implications of food–drug interactions with small-molecule kinase inhibitors. Lancet Oncol. 2020;21(5):e265–79. https://doi.org/10.1016/s1470-2045(20)30069-3.
Yan JH. Food effect on oral bioavailability: old and new questions. Clin Pharmacol Drug Dev. 2017;6(4):323–30. https://doi.org/10.1002/cpdd.351.
Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K, Stead A, Beelen AP, Lewis LD. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol. 2009;27(8):1191–6. https://doi.org/10.1200/jco.2008.18.3285.
Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–56. https://doi.org/10.2165/00003088-200443150-00005.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. https://doi.org/10.1007/s11095-004-9004-4.
Xu H, O’Gorman M, Boutros T, Brega N, Kantaridis C, Tan W, Bello A. Evaluation of crizotinib absolute bioavailability, the bioequivalence of three oral formulations, and the effect of food on crizotinib pharmacokinetics in healthy subjects. J Clin Pharmacol. 2015;55(1):104–13. https://doi.org/10.1002/jcph.356.
Tugnait M, Gupta N, Hanley MJ, Venkatakrishnan K, Sonnichsen D, Kerstein D, Dorer DJ, Narasimhan N. The effect of a high-fat meal on the pharmacokinetics of brigatinib, an oral anaplastic lymphoma kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev. 2019;8(6):734–41. https://doi.org/10.1002/cpdd.641.
Xu H, O’Gorman MT, Nepal S, James LP, Ginman K, Pithavala YK. Phase 1 study evaluating the effects of the proton pump inhibitor rabeprazole and food on the pharmacokinetics of lorlatinib in healthy participants. Clin Pharmacol Drug Dev. 2021;10(11):1395–404. https://doi.org/10.1002/cpdd.1000.
Meneses-Lorente G, Bentley D, Guerini E, Kowalski K, Chow-Maneval E, Yu L, Brink A, Djebli N, Mercier F, Buchheit V, Phipps A. Characterization of the pharmacokinetics of entrectinib and its active M5 metabolite in healthy volunteers and patients with solid tumors. Invest New Drugs. 2021;39(3):803–11. https://doi.org/10.1007/s10637-020-01047-5.
Morcos PN, Guerini E, Parrott N, Dall G, Blotner S, Bogman K, Sturm C, Balas B, Martin-Facklam M, Phipps A. Effect of food and esomeprazole on the pharmacokinetics of alectinib, a highly selective ALK inhibitor, in healthy subjects. Clin Pharmacol Drug Dev. 2017;6(4):388–97. https://doi.org/10.1002/cpdd.296.
Morcos PN, Parrott N, Banken L, Timpe C, Lindenberg M, Guerini E, Dall G, Bogman K, Sturm C, Zeaiter A, Martin-Facklam M, Phipps A. Effect of the wetting agent sodium lauryl sulfate on the pharmacokinetics of alectinib: results from a bioequivalence study in healthy subjects. Clin Pharmacol Drug Dev. 2017;6(3):266–79. https://doi.org/10.1002/cpdd.299.
Lau YY, Gu W, Lin T, Song D, Yu R, Scott JW. Effects of meal type on the oral bioavailability of the ALK inhibitor ceritinib in healthy adult subjects. J Clin Pharmacol. 2016;56(5):559–66. https://doi.org/10.1002/jcph.619.
Shao R, Chen W, Ruan Z, Yang D, Chen W, Li H, Lou H, Chen J, Jiang B. Effects of food on the pharmacokinetics of ensartinib in healthy Chinese subjects. Clin Exp Pharmacol Physiol. 2022;49(3):360–9. https://doi.org/10.1111/1440-1681.13611.
Acknowledgements
Editorial support for this manuscript was provided by Jake Burrell PhD (Rude Health Consulting) and paid for by Fosun Pharma.
Funding
This study was funded by Shanghai Fosun Pharmaceutical Development Co., Ltd and Wanbang Biopharmaceuticals.
Author information
Authors and Affiliations
Contributions
Wei Hu, Lei Diao, Huiling Qin, Yan Tan, Ai-Min Hui, Yongchun Zhou, Zhuli Wu and Juan Sun participated to the study design, study conduct and the review and approval of this article. Xiao Xiang and Jingjun Qiu participated in the analysis and interpretation of data as well as the review and approval of the article.
Corresponding author
Ethics declarations
Conflict of Interest
Yan Tan, Lei Diao, Zhuli Wu, Juan Sun, Xiao Xiang and Jingjun Qiu, are employees of Shanghai Fosun Pharmaceutical Industrial Development CO., Ltd. Yongchun Zhou is an employee of Wanbang Biopharmaceuticals. Ai-Min Hui is an employee of EnCureGen Pharma. Huiling Qin and Wei Hu have no conflicts of interest to declare.
Ethics Approval
The protocol for this study was approved by the Ethics Review Board at The Second Hospital of Anhui Medical University (approval number: CTR20212829). All subjects provided written, informed consent before entering this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Qin, H., Tan, Y., Diao, L. et al. Effect of High-Fat Food on the Pharmacokinetic Profile and Safety of SAF-189s, an ALK/ROS1 Inhibitor, in Healthy Chinese Adults. Drugs R D 23, 465–473 (2023). https://doi.org/10.1007/s40268-023-00446-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00446-2