Abstract
Introduction
Breakthrough pain (BTP) management in patients with cancer is challenging, especially in the elderly. However, no studies examining the influence of age on BTP medication have been conducted. The aim of this work was to investigate the effect of sublingual fentanyl tablets (SFTs) in terms of efficacy, safety, and quality of life in two age categories.
Methods
We performed age subgroup analyses (<65 and ≥65 years) from a recently completed study conducted in Spain. Pain intensity (PI), onset of pain relief, frequency and duration of BTP episodes, and adverse events (AEs) were assessed at 3, 7, 15, and 30 days. Health-status instruments used were the Short Form 12, version 2 (SF-12v2) questionnaire, and the Hospital Anxiety and Depression Scale (HADS-A and HADS-D).
Results
Twenty-six patients were aged <65 years and 54 were aged ≥65 years. SF-12v2 scores did not enhance significantly from baseline. HADS scores and PI decreased significantly at the end of the study, particularly in younger patients (HADS-A: 19.05 vs. 14.41%; HADS-D: 21.35 vs. 18.57%; PI: 67.23 vs. 56.30%). Onset of analgesia began in 2–5 min in 63.3% of subjects aged <65 years and in 36.4% of subjects aged >65 years. Most patients experienced one to five daily episodes after 30 days, and <5% needed a treatment change. AEs were less frequently reported in older individuals (20.5 vs. 36.4%).
Conclusion
Age subgroup analyses suggest that SFTs are an effective and safe treatment for the management of BTP in cancer patients of all ages. SFTs may offer a well-tolerated and efficient option to control cancer BTP in the elderly.
Similar content being viewed by others
In this paper, we report on the effect of a sublingual formulation of fentanyl in the management of breakthrough pain in patients with cancer according to age, in terms of efficacy, safety, pain relief, and quality of life. This is significant because this medication could represent an efficient treatment option to control the transient exacerbation of pain in elderly patients with cancer. |
1 Introduction
Pain is one of the most common problems in patients with cancer, especially in the elderly. Existing studies indicate that older people with cancer pain are poorly managed [1], and they often present more complex clinical problems than younger people. As a consequence, it becomes necessary to understand how to most effectively treat cancer-associated pain in an increasingly aging population.
Patients with cancer, in addition to persistent pain, may also experience breakthrough pain (BTP), a transient exacerbation of pain that occurs on a background of chronic, persistent pain in patients receiving chronic opioid therapy [2, 3]. BTP is generally characterized by short, severe occurrences of pain, which usually last from several to tens of minutes, and a mean duration of 45–60 min, with several episodes per day. Along with background pain, BTP produces significant suffering and anxiety, and is frequently associated with depression, leading to a poorer quality of life (QoL) and mental health. Therefore, BTP represents a significant treatment challenge and demands a fast-acting therapeutic regimen to control the pain and its impact on a patient’s QoL.
The selection of the most suitable pharmacological option and route of administration should be determined by the duration, intensity, and onset of BTP. In oncology, opioids have recently become the cornerstone of cancer pain management [4]. Management of BTP via transmucosal and sublingual opioid formulations, such as fentanyl preparations, provides a non-invasive mechanism for immediate drug absorption, high bioavailability, and rapid onset of pain relief compared with traditional oral dosing [5, 6]. Sublingual fentanyl tablets (SFTs), which allow for rapid absorption of fentanyl through the sublingual mucosa, have been shown to be effective and well-tolerated in controlling BTP in cancer patients. Additionally, they have been proved to potentially enhance QoL [7,8,9].
However, age subgroup analysis of efficacy or adverse effects of BTP medications has been rarely reported in the literature and deserve further examination [5, 10]. To date, no studies exist examining the effect of age on SFT dosing on pain symptoms and QoL.
The aim of this article was to provide data of an age subgroup analysis from a recently completed trial contributing to the assessment of the effect of SFT in patients with cancer pain. Eighty-one patients were included in the study (26 aged <65 years and 54 aged ≥65 years). Data from the study have been previously published [11]. We specifically investigated patients’ outcomes in terms of efficacy, safety, pain relief, and QoL.
2 Methods
2.1 Study Design and Population
Study design and eligibility criteria have been previously described in detail elsewhere [11]. This was a multicenter, prospective, observation, post-authorization, open-label study conducted at nine pain units in Catalonia and the Balearic Islands in Spain between March and December 2013. Patients were eligible if they had a confirmed diagnosis of cancer and were regularly experiencing episodes of BTP with a value of ≥6 measured using a visual analog scale (VAS). All patients were receiving a fixed-dose schedule of opioids equivalent to oral morphine of at least 60 mg/day, or transdermal fentanyl 25 μg/h, oral oxycodone 30 mg/day, oral hydromorphone 8 mg/day, oral oxymorphone 25 mg/day, or an equianalgesic dose of any other opioid. Participants were fully informed of the study and provided signed written informed consent.
At the initial screening visit, patients provided data on their baseline health, treatment, pain, and QoL. All outcomes were assessed at 3 (visit 1), 7 (visit 2), 15 (visit 3), and 30 days (end of study) after starting the treatment. For each BTP episode, patients self-administered SFT (Abstral®, Kyowa Kirin Farmacéutica SLU, Madrid, Spain). The initial dose of SFT was determined by the clinician on the basis of prior treatment for BTP and with consideration of the opioid dose for background pain. The dose was then titrated up to a successful analgesia (100, 200, 300, 400, 600, 800 μg). Changes in the dose of SFT were recorded throughout the study.
2.2 Efficacy and Tolerability Assessments
The instruments used to evaluate the patients’ QoL included the Short Form 12 (version 2) questionnaire (SF-12v2), with a physical health composite score (PCS) and a mental health score (MCS) [12, 13], and the Hospital Anxiety and Depression Scale (HADS), consisting of seven statements on the anxiety subscale (HADS-A) and seven on the depression subscale (HADS-D) [14, 15]. Patients rated their pain intensity (PI) using an 11-point numerical rating scale, from 0 (no pain) to 10 (worst pain imaginable). Pain relief was assessed by asking patients to select from a list of time intervals the ‘time to first effect’ and the ‘time to maximum effect’ following administration of SFTs. Additional endpoints were reported by each patient, including number of irruptive pain episodes and duration of each episode at each assessment. Safety and tolerability were assessed based on patients’ and clinicians’ reports of adverse events (AEs).
2.3 Statistical Analysis
Subgroup analyses were performed according to age (<65 vs. ≥65 years). Demographics and disease-related features were analyzed descriptively using frequencies, means and standard deviations (SDs), as appropriate. The SF-12 physical and mental health component summary scores were computed as normalized scores (mean = 50, SD = 10). Statistical analyses were performed with a paired two-tailed t-test, and p-values <0.05 were considered statistically significant, with no adjustments for multiplicity. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
3 Results
Between March and December 2013, 81 patients were enrolled, with 69 completing the 30-day observation period. The mean age was 69.7 years (range 40–91 years), and 58.0% of patients were female. Twenty-six individuals were younger than 65 years of age, and 54 were older than 65 years of age (one patient’s birthday data was not available). Details on other patient demographics and baseline characteristics have been previously summarized elsewhere [11].
3.1 Quality-of-Life Outcomes
QoL scores did not show significant improvement from baseline scores and subgroups, and, regarding mental scores, although the changes observed between groups at the end of the study were not statistically significant, a significant reduction from baseline scores was observed in both subgroups.
3.2 Pain-Intensity Outcomes
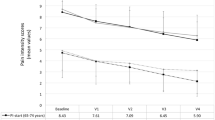
Table 1 shows the pain-intensity outcomes throughout the study for each subgroup of patients. Self-reported levels of PI improved significantly compared with baseline for all assessment points and both subgroups (p < 0.05). For each assessment point, improvement of PI was higher in patients aged <65 years. At the end of the study, PI decreased from 5.95 to 1.95 (67.23% reduction) in the subgroup of patients aged <65 years, and from 6.11 to 2.67 (56.30% reduction) in the subgroup of patients aged ≥65 years.
3.3 Additional Outcomes
Most patients experienced one to five daily episodes at the end of the study, without differences between subgroups. The onset of analgesia began before 5 min in 63.6% of subjects (14 of 22 patients) under 65 years of age and in 36.4% of subjects (16 of 44 patients) over 65 years of age (p = 0.0359). Patients reported that the time to first effect following administration of SFTs at the end of the study was within 10 min in 86.4% (19 of 22 patients) and 72.7% (32 of 44 patients) of cases (p = 0.2127) <65 and ≥65 years of age, respectively (Fig. 1).
At all clinical visits, patients above 65 years of age presented a higher mean number of irruptive pain episodes compared with younger patients (18.12 ± 17.67 vs. 7.21 ± 5.43 at the end of the study) (Table 2).
The percentage of cumulative proportion of patients (%) versus the average duration of each BTP episode (minutes) is shown in Fig. 2. In general, patients under 65 years of age experienced a shorter duration of BTP events (BTP episodes lasted less than 5 min in 36.4% [8 of 22] of patients in this subgroup, compared with 15.9% [7 of 44] in older patients; p = 0.0616). A proportion of 86.4% (19 of 22) of younger patients and 65.9% (29 of 44) of older patients suffered BTP episodes for a period of <15 min (p = 0.0786).
The study medication management was examined at each visit based on patients’ and clinicians’ reports. Older patients seemed to need a dose increase more frequently than younger patients. However, differences were not statistically significant. The average dose per day at the end-of-study visit was 272.73 μg (mean ± SD 272.73 ± 138.64 μg) in patients aged <65 years and 357.14 μg (mean ± SD 357.14 ± 239.05 μg) in patients aged ≥65 years.
A subanalysis of the study was made according to sex. Different variables at all visits were evaluated but no significant differences were observed between men and women.
3.4 Safety Endpoints
At the end-of-study visit, AEs were reported in a minority of individuals—8 and 9 in the <65 and ≥65 years subgroups, respectively (Table 3). The most frequently reported AE was constipation, followed by somnolence, nausea, vomiting, and skin disorders. Most AEs were considered mild or moderate in severity.
4 Discussion
BTP affects a large proportion of cancer patients and its treatment is challenging, particularly in elderly patients, because of the increased risk of therapeutic complications [5]. Age-based disparities in the treatment of BTP have not been previously described. The current age subgroup analysis of a multicenter prospective study aimed to investigate the management of BTP in cancer patients via SFTs (a sublingual opioid formulation) in terms of efficacy, tolerability, pain relief, and QoL in two age categories (<65 and ≥65 years).
Assessment of the impact of BTP treatment with SFTs on health-related QoL through changes on the depression and anxiety subscales of the HADS questionnaire revealed some differences between the two groups of patients. Although there was a significant improvement in anxiety and depression in both groups, score reduction was higher in patients under 65 years of age. Moreover, a cut-off point of ≥8 for the HADS-A or HADS-D subscales has been described as an indicator for clinically significant anxiety or depression [14, 16], and only younger patients presented scores below this threshold value at the end of the study. These results reflect that managing BTP with SFTs can reduce the negative effects of BTP on QoL, particularly in patients under 65 years of age. The results of this subgroup analysis were not consistent with those observed in the overall population, where changes in both PCS and MCS scores demonstrated significant improvements [11]. Differences could be due to the smaller number of participants in both age subgroups.
Improvement of PI was higher in patients <65 years of age, suggesting better efficacy results in these subjects. Despite most patients experiencing one to five episodes of BTP per day at the end of the study, patients above 65 years of age presented a higher number of irruptive pain episodes. A lower percentage of these patients experienced the first effect of analgesia in 2–5 min, following administration of SFTs, compared with younger patients (36.4 vs. 56.0%, respectively). The data also showed that individuals above 65 years of age experienced a longer duration of BTP events, and the dose of SFTs they were taking was more frequently increased.
All these observations indicate that substantial differences exist between the two groups when comparing the number and duration of BTP episodes. Nonetheless, the use of SFTs in both groups was effective. The rapid onset of action of SFTs (inferior to 5 min) in a high percentage of younger patients, who reported less and shorter BTP episodes than older individuals, closely mirrored the temporal pattern of BTP episodes. In general, the elderly population are more sensitive to opioids and experience a greater frequency of decreased hepatic, renal, or cardiac function. For this reason, dose selection for an elderly patient usually starts at the low end of the dosing range and increases gradually to obtain an appropriate balance between management of pain and opioid-related adverse reactions.
Surprisingly, age seemed to affect the tolerability and safety of SFTs since patients above 65 years of age reported less AEs than younger patients (36.4 and 20.5% in subjects aged <65 and ≥65 years, respectively). These results are interesting since the older patient is considered to be more susceptible to be affected by opioid toxicity due to the physiologic changes associated with aging [17]. Adverse effects generally increase in the setting of multimorbidity, polypharmacy, and physiologic vulnerability of older patients, who require lower doses than younger patients to obtain a successful analgesia [18]. However, our study suggests that the use of SFTs in these patients does not lead to an increase of AEs. Indeed, very few individuals needed to switch to alternative opioids.
SFTs may offer a well-tolerated and efficient option to control BTP in elderly patients with cancer. These conclusions are in accordance with some considerations recently published on the management of breakthrough cancer pain in the elderly [19]. This review proposes the use of fentanyl preparations, designed to provide a rapid and effective analgesia, as the first treatment option of BTP in older patients.
The potential limitations of this study should be considered. The small sample size used for data analysis, and thus its low power, may have biased the observed differences between age subgroups. The differences found could potentially become statistically significant in a larger population. Other limitations include the subjective nature of pain and well-being, and the instruments used to assess the endpoints of the study. Thus, it becomes complex to compare outcomes between groups of patients.
Despite these limitations, the present findings provide significant implications for the management of BTP with SFTs for patients with cancer pain according to age, as well as relevant evidence for improving health and QoL in the elderly. Age subgroup analyses of efficacy or adverse effects of BTP medications have not been previously reported in the literature [17, 20], therefore further investigation of the comparative effectiveness of BTP therapies would be of interest. On the other hand, studies to evaluate the role of opioid analgesics in older adults with pain have been previously published [21].
References
Mercadante S, Arcuri E. Pharmacological management of cancer pain in the elderly. Drugs Aging. 2007;24(9):761–76.
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41(3):273–81.
Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–8.
Starr TD, Rogak LJ, Passik SD. Substance abuse in cancer pain. Curr Pain Headache Rep. 2010;14(4):268–75. doi:10.1007/s11916-010-0118-6.
Pautex S, Vogt-Ferrier N, Zulian GB. Breakthrough pain in elderly patients with cancer: treatment options. Drugs Aging. 2014;31(6):405–11. doi:10.1007/s40266-014-0181-5 (review).
Lennernäs B, Frank-Lissbrant I, Lennernäs H, Kälkner KM, Derrick R, Howell J. Sublingual administration of fentanyl to cancer patients is an effective treatment for breakthrough pain: results from a randomized phase II study. Palliat Med. 2010;24(3):286–93. doi:10.1177/0269216309356138 (Epub 16 Dec 2009).
Chwieduk CM, McKeage K. Fentanyl sublingual: in breakthrough pain in opioid-tolerant adults with cancer. Drugs. 2010;70(17):2281–8. doi:10.2165/11200910-000000000-00000.
Rauck RL, Tark M, Reyes E, Hayes TG, Bartkowiak AJ, Hassman D, Nalamachu S, Derrick R, Howell J. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin. 2009;25(12):2877–85. doi:10.1185/03007990903368310.
Überall MA, Müller-Schwefe GH. Sublingual fentanyl orally disintegrating tablet in daily practice: efficacy, safety and tolerability in patients with breakthrough cancer pain. Curr Med Res Opin. 2011;27(7):1385–94. doi:10.1185/03007995.2011.583231 (Epub 2011 May 11).
Mercadante S, Giarratano A. Assessing age and gender in studies of breakthrough pain medications. Curr Med Res Opin. 2014;30:1353–6.
Guitart J, Vargas MI, De Sanctis V, Folch J, Salazar R, Fuentes J, Coma J, Ferreras J, Moya J, Tomás A, Estivill P, Rodelas F, Jiménez AJ. Sublingual fentanyl tablets for relief of breakthrough pain in cancer patients and association with quality-of-life outcomes. Clin Drug Investig. 2015;35(12):815–22. doi:10.1007/s40261-015-0344-0 (Erratum in: Clin Drug Investig 2016).
Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
Schmidt S, Vilagut G, Garin O, Cunillera O, Tresserras R, Brugulat P, Mompart A, Medina A, Ferrer M, Alonso J. Reference guidelines for the 12-Item Short-Form Health Survey version 2 based on the Catalan general population. Med Clin (Barc). 2012;139(14):613–25. doi:10.1016/j.medcli.2011.10.024 (Epub 11 Jan 2012).
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Herrero MJ, Blanch J, Peri JM, De Pablo J, Pintor L, Bulbena A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry. 2003;25(4):277–83.
Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi:10.1016/S0022-3999(01)00296-3.
Mercadante S, Aielli F, Masedu F, Valenti M, Ficorella C, Porzio G. Pain characteristics and analgesic treatment in an aged adult population: a 4-week retrospective analysis of advanced cancer patients followed at home. Drugs Aging. 2015;32(4):315–20. doi:10.1007/s40266-015-0253-1.
Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient a clinical review. JAMA. 2014;312(8):825–36. doi:10.1001/jama.2014.9405.
Cabezón-Gutiérrez L, Viloria-Jiménez MA, Pérez-Cajaraville J, Álamo-González C, López-Trigo JA, Gil-Gregorio P. en representación del Comité de Expertos del Dolor de la Sociedad Española de Geriatría y Gerontología. [Breakthrough cancer pain in the elderly]. [Article in Spanish]. Rev Esp Geriatr Gerontol. 2016;. doi:10.1016/j.regg.2016.10.003 (Epub ahead of print).
Mercadante S, Mercadante A, Aielli F. Effect of aging on pain relief in the older cancer patients: pharmacokinetic and pharmacodynamic aspects. Expert Opin Drug Metab Toxicol. 2016;12(7):711–3. doi:10.1517/17425255.2016.1152263 (Epub 27 Feb 2016).
Greene Naples J, Gellad WF, Hanlon JT. Managing pain in older adults: the role of opioid analgesics. Clin Geriatr Med. 2016;32(4):725–35. doi:10.1016/j.cger.2016.06.006.
Acknowledgements
The authors thank Blanca Martínez-Garriga, who provided medical writing assistance on behalf of Trialance (http://www.trialance.com).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Jordi Guitart, MD declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
María Isabel Vargas declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Vicente De Sanctis, MD declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Jordi Folch declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Rafael Salazar declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
José Fuentes declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Joan Coma declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Julia Ferreras declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Jordi Moya declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Albert Tomás declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Pere Estivill declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Francisco Rodelas declares he has no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Antonio Javier Jiménez works at Medical Department of Kyowa Kirin Farmacéutica, S.L.U.
Almudena Sanz works at Medical Department of Kyowa Kirin Farmacéutica, S.L.U.
Funding
This study was funded by Kyowa Kirin Farmacéutica, S.L.U.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Guitart, J., Vargas, M.I., De Sanctis, V. et al. Breakthrough Pain Management with Sublingual Fentanyl Tablets in Patients with Cancer: Age Subgroup Analysis of a Multicenter Prospective Study. Drugs R D 17, 419–425 (2017). https://doi.org/10.1007/s40268-017-0198-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-017-0198-4