Abstract
Background and objective
Although cannabinoid-based medications are increasingly used by older adults, their safety and tolerability in this age group remain unclear. The purpose of this systematic review was to examine the safety and tolerability of cannabinoid-based medications by conducting a meta-analysis of open-label observational studies of cannabinoid-based medications for all indications in individuals with a mean age of ≥50 years.
Methods
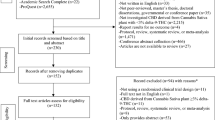
A systematic search was conducted on PubMed, PsycINFO, MEDLINE, EMBASE and CINHAL. Study quality was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. We included studies that (a) were published from 1990 onwards; (b) included older adults (mean age ≥50 years); and (c) provided data on the safety and tolerability of medical cannabinoids. Data were pooled using a random-effects approach. Risk of adverse events, serious adverse events and withdrawals was computed as the incidence rate (IR). Separate analyses were conducted by the cannabinoid-based medication used, for delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD) and a combination of THC and CBD (THC:CBD).
Results
Thirty-eight studies were identified (THC = 23; CBD = 6; THC:CBD = 9; N = 2341, mean age: 63.19 ± 8.08 years, men: 53.86%). THC had a very low incidence of all-cause and treatment-related adverse events (IR: 122.18, 95% confidence interval [CI] 38.23–253.56; IR: 84.76, 95% CI 0.13–326.01, respectively) and negligible serious adverse events (IR = 0). Similar IRs for CBD (all cause, IR: 111.91, 95% CI 1.24–495.93; treatment related, IR: 1.76, 95% CI 4.63–23.05) and no serious adverse events (IR = 0). CBD was not associated with a risk of treatment-related withdrawals. THC had a low risk of all-cause and treatment-related withdrawals (IR: 25.18, 95% CI 12.35–42.52; IR: 7.83, 95% CI 3.26–14.38, respectively). The THC:CBD treatment had a low risk of all-cause and treatment-related adverse events (IR: 100.72, 95% CI 0.25–383.00; IR: 55.38, 95% CI 8.61–142.80, respectively), but reported a risk of all-cause and treatment-related serious adverse events (IR: 21.32, 95% CI 0.18–93.26; IR: 3.71, 95% CI 0.21–11.56, respectively), and all-cause and treatment-related withdrawals (IR: 78.63, 95% CI 17.43–183.90; IR: 34.31, 95% CI 6.09–85.52, respectively). Significant heterogeneity (I2 >55%) was present in most analyses.
Conclusions
Although cannabinoid-based medications were generally safe and acceptable to adults aged over 50 years, these estimates are limited by the lack of a control condition and considerable heterogeneity. Nevertheless, they complement and are consistent with comparable evidence from randomised controlled trials.

Similar content being viewed by others
References
Beedham W, Sbai M, Allison I, Coary R, Shipway D. Cannabinoids in the older person: a literature review. Geriatrics (Basel). 2020;5(1):2.
Sznitman SR, Vulfsons S, Meiri D, Weinstein G. Medical cannabis and cognitive performance in middle to old adults treated for chronic pain. Drug Alcohol Rev. 2020;40(2):272–80.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73.
Pertwee R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215.
Leweke FM, Mueller JK, Lange B, Rohleder C. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604–12.
Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorganic Med Chem. 2015;23(7):1377–85.
Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66(4):442–51.
Bhattacharyya S, Wilson R, Appiah-Kusi E, O’Neill A, Brammer M, Perez J, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiat. 2018;75(11):1107–17.
Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiarty. 2019;6(12):995–1010.
Han BH, Sherman S, Mauro PM, Martins SS, Rotenberg J, Palamar JJ. Demographic trends among older cannabis users in the United States, 2006–13. Addiction. 2017;112(3):516–25.
Engels FK, de Jong FA, Mathijssen RH, Erkens JA, Herings RM, Verweij J. Medicinal cannabis in oncology. Eur J Cancer. 2007;43(18):2638–44.
Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. Pharm Ther. 2017;42(3):180.
Beauchet O. Medical cannabis use in older patients: update on medical knowledge. Maturitas. 2018;118:56–9.
Stoner S. Marijuana use by older adults. Alcohol & Drug Abuse Institute, University of Washington. 2016. http://LearnAboutMarijuanaWA.org/factsheets/olderadults.htm.
Bobitt J, Qualls SH, Schuchman M, Wickersham R, Lum HD, Arora K, et al. Qualitative analysis of cannabis use among older adults in Colorado. Drugs Aging. 2019;36(7):655–66.
Baumbusch J, Sloan YI. Exploring new use of cannabis among older adults. Clin Gerontol. 2021;44(1):25–31.
Bar-Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37–43.
Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31(11):1553–60.
Barrie AM, Gushue AC, Eskander RN. Dramatic response to laetrile and cannabidiol (CBD) oil in a patient with metastatic low grade serous ovarian carcinoma. Gynecol Oncol Rep. 2019;29:10–2.
Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195–220.
Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6(1–2):24–30.
Bidwell LC, Ellingson JM, Karoly HC, Yorkwilliams SL, Hitchcock LN, Tracy BL, et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiat. 2020;77(8):787–96.
Alton Croker 3rd J, Bobitt JL, Arora K, Kaskie B. Assessing health-related outcomes of medical cannabis use among older persons: findings from Colorado and Illinois. Clin Gerontol. 2021;44(1):66–79.
Jadoon KA, Tan GD, O’Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight. 2017;2(12):e93760.
Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139–54.
Leys F, Raccagni C, Sidoroff V, Seppi K, Fanciulli A, Wenning GK. Effects of self-administered cannabidiol in a patient with multiple system atrophy. Clin Autonom Res. 2020;30(4):355–6.
Peball M, Krismer F, Knaus HG, Djamshidian A, Werkmann M, Carbone F, et al. Non-motor symptoms in Parkinson’s disease are reduced by nabilone. Ann Neurol. 2020;88(4):712–22.
Ruthirakuhan M, Herrmann N, Andreazza AC, Verhoeff NPL, Gallagher D, Black SE, et al. Agitation, oxidative stress, and cytokines in Alzheimer disease: biomarker analyses from a clinical trial with nabilone for agitation. J Geriatr Psych Neurol. 2020;33(4):175–84.
Spiera R, Hummers L, Chung L, Frech TM, Domsic R, Hsu V, et al. Safety and efficacy of lenabasum in a phase II, randomized, placebo-controlled trial in adults with systemic sclerosis. Arthritis Rheum. 2020;72(8):1350–60.
Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–30.
Kamrul R, Bunka D, Crawley A, Schuster B, LeBras M. Navigating cannabinoid choices for chronic neuropathic pain in older adults: potholes and highlights. Can Family Phys. 2019;65(11):807–11.
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):50–2.
van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res. 2014;14:56–64.
Wang T, Collet J-P, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178(13):1669–78.
Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–74.
O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2021;51(4):596–606.
Velayudhan L, McGoohan K, Bhattacharyya S. Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: a systematic review and meta-analysis. PLOS Med. 2021;18(3):e1003524.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JP. Critical interpretation of Cochran’s Q test depends on power and prior assumptions about heterogeneity. Res Synth Methods. 2010;1(2):149–61.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Udow SJ, Freitas ME, Fox SH, Lang AE. Exacerbation of psychosis triggered by a synthetic cannabinoid in a 70-year-old woman with Parkinson disease. CMAJ. 2018;190(2):E50–2.
Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153(10):2073–82.
Sinha A, Singh P, Kupfer Y. Dronabinol-induced acute altered mental status in an elderly patient. Am J Ther. 2018;25(4):e502–3.
Cunetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, Orihuela S. Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc. 2018;50(2):461–4.
Tayo B, Taylor L, Sahebkar F, Morrison G. A phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet. 2020;59(6):747–55.
Galeano D, Li S, Gerstein M, Paccanaro A. Predicting the frequencies of drug side effects. Nat Commun. 2020;11(1):4575.
Brenneisen R, Egli A, Elsohly M, Henn V, Spiess Y. The effect of orally and rectally administered Δ 9-tetrahydrocannabinol on spasticity: a pilot study with 2 patients. Int J Clin Pharmacol Ther. 1996;34:446–52.
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manag. 2013;46(2):207–18.
Guzman M, Duarte MJ, Blazquez C, Ravina J, Rosa MC, Galve-Roperh I, et al. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203.
Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis‐based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3(3):CD012182.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014;(3):CD009270.
Walitt B, Klose P, Fitzcharles MA, Phillips T, Häuser W. Cannabinoids for fibromyalgia. Cochrane Database Syst Rev. 2016;7(7):CD011694.
Guina J, Merrill B. Benzodiazepines I: upping the care on downers: the evidence of risks, benefits and alternatives. J Clin Med. 2018;7(2):17.
Guina J, Rossetter SR, DeRhodes BJ, Nahhas RW, Welton RS. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281–303.
Ravindran LN, Stein MB. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res. 2009;1293:24–39.
Hoggart B, Ratcliffe S, Ehler E, Simpson KH, Hovorka J, Lejcko J, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol. 2015;262(1):27–40.
Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice: results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71(5–6):271–9.
Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013;260(1):285–95.
Zajac DM, Sikkema SR, Chandrasena R. Nabilone for the treatment of dementia-associated sexual disinhibition. Prim Care Companion CNS Disord. 2015;17(1). https://doi.org/10.4088/PCC.14l01695.
Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J Alzheimers Dis. 2016;51(1):15–9.
Nelson K, Walsh D, Deeter P, Sheehan F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliat Care Spring. 1994;10(1):14–8.
Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage. 2005;30(6):493–5.
Marinelli L, Mori L, Canneva S, Colombano F, Curra A, Fattapposta F, et al. The effect of cannabinoids on the stretch reflex in multiple sclerosis spasticity. Int Clin Psychopharmacol. 2016;31(4):232–9.
Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis-based medicines: a novel paradigm and treatment for non-uremic calciphylaxis leg ulcers: an open label trial. Int Wound J. 2020;17(5):1508–16.
Heim B, Bajaj S, Marzi RD, Mangesius S, Djamshidian A, Poewe W, et al. M6 Nabilone in huntington’s disease: a case series of five patients. J Neurol Neurosurg Psychiatry. 2016;87(Suppl. 1):A103.
Defrancesco M, Hofer A. Cannabinoid as beneficial replacement therapy for psychotropics to treat neuropsychiatric symptoms in severe Alzheimer’s dementia: a clinical case report. Front Psychiatry. 2020;11:413.
Wade D, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006;12:639–45.
Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23(8):979–83.
Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78(17):1791–804.
Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. 2020;61(6):1090–8.
Rong C, Carmona NE, Lee YL, Ragguett R-M, Pan Z, Rosenblat JD, et al. Drug-drug interactions as a result of co-administering Δ9-THC and CBD with other psychotropic agents. Expert Opin Drug Saf. 2018;17(1):51–4.
Zhu J, Peltekian KM. Cannabis and the liver: things you wanted to know but were afraid to ask. Toronto (ON): University of Toronto Press; 2019.
Alsherbiny MA, Li CG. Medicinal cannabis: potential drug interactions. Medicines (Basel). 2018;6(1):3.
Brown JD, Winterstein AG. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8(7):989.
Brown GW, Bellnier TJ, Janda M, Miskowitz K. DELTA-9-tetrahydrocannabinol dose increase leads to warfarin drug interaction and elevated INR. J Am Pharm Assoc (2003). 2021;61(1):e57–60.
Flach AJ. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans Am Ophthalmol Soc. 2002;100:215–22; discussion 222–4.
Attal N, Brasseur L, Guirimand D, Clermond-Gnamien S, Atlami S, Bouhassira D. Are oral cannabinoids safe and effective in refractory neuropathic pain? Eur J Pain. 2004;8(2):173–7.
Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology. 2006;185(4):524–8.
Wilson MG, Philpot C, Morley J. Anorexia of aging in long term care: is dronabinol an effective appetite stimulant? A pilot study. J Nutr Health Aging. 2007;11(2):195–8.
Maida V. Nabilone for the treatment of paraneoplastic night sweats: a report of four cases. J Palliat Med. 2008;11(6):929–34.
Passmore MJ. The cannabinoid receptor agonist nabilone for the treatment of dementia-related agitation. Int J Geriatr Psychiatry. 2008;23(1):116–7.
Bestard JA, Toth CC. An open-label comparison of nabilone and gabapentin as adjuvant therapy or monotherapy in the management of neuropathic pain in patients with peripheral neuropathy. Pain Pract. 2011;11(4):353–68.
Joerger M, Wilkins J, Fagagnini S, Baldinger R, Brenneisen R, Schneider U, et al. Single-dose pharmacokinetics and tolerability of oral delta-9- tetrahydrocannabinol in patients with amyotrophic lateral sclerosis. Drug Metab Lett. 2012;6(2):102–8.
Amanullah S, MacDougall K, Sweeney N, Coffin J, Cole J. Synthetic cannabinoids in dementia with agitation: case studies and literature review. Clin Neuropsychiatry. 2013;10:142–7.
Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–9.
Poli P, Crestani F, Salvadori C, Valenti I, Sannino C. Medical cannabis in patients with chronic pain: effect on pain relief, pain disability, and psychological aspects: a prospective non randomized single arm clinical trial. Clin Ter. 2018;169(3):e102–7.
Abuhasira R, Ron A, Sikorin I, Novack V. Medical cannabis for older patients: treatment protocol and initial results. J Clin Med. 2019;8(11):1819.
Fallon MT, Albert Lux E, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11(3):119–33.
Crescioli G, Lombardi N, Bettiol A, Menniti-Ippolito F, Da Cas R, Parrilli M, et al. Adverse events following cannabis for medical use in Tuscany: an analysis of the Italian Phytovigilance database. Br J Clin Pharmacol. 2020;86(1):106–20.
Chagas MH, Eckeli AL, Zuardi AW, Pena-Pereira MA, Sobreira-Neto MA, Sobreira ET, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
SB and LV received funding from Parkinson’s UK (grant number G-1901). SB received a grant from the National Institute of Health Research Efficacy and Mechanism Evaluation scheme (UK) [grant no. 16/126/53]. The authors acknowledge support from the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No other disclosures were reported.
Conflicts of interest
Sara Pisani, Katie McGoohan, Latha Velayudhan and Sagnik Bhattacharyya have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
This publication is a systematic review and meta-analysis, and as such it includes data that have already been published. The data that support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
Author contributions
Concept and design: LV, SB. Acquisition, analysis or interpretation of the data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: SP, SB. Obtained funding: SB. Supervision: LV, SB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pisani, S., McGoohan, K., Velayudhan, L. et al. Safety and Tolerability of Natural and Synthetic Cannabinoids in Older Adults: A Systematic Review and Meta-Analysis of Open-Label Trials and Observational Studies. Drugs Aging 38, 887–910 (2021). https://doi.org/10.1007/s40266-021-00882-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-021-00882-2