Abstract
Background
Tramadol is frequently used in geriatric patients; however, pharmacokinetic (PK) publications on tramadol and O-desmethyltramadol (ODM) in elderly patients are rare.
Objective
Our objective was to characterize the PK of tramadol and ODM, including absorption processes and covariates for tramadol, in elderly and young subjects after single-dose administration of 200-mg extended-release tablets.
Methods
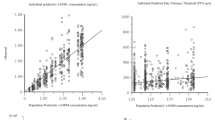
We conducted a PK study in 15 elderly (aged ≥75 years) subjects with mild renal insufficiency and 20 young (18–40 years) subjects; blood and urine samples were collected for 48 h post-dose. Non-compartmental analysis (NCA) of each tramadol and ODM enantiomer included area under the concentration–time curve (AUC), terminal elimination rate (k el), total body clearance, volume of distribution (V area/ F), and renal clearance (Clr0–48). A one-compartment population model of total tramadol concentration was parameterized with clearance (CL/F), volume of distribution (V/F), and mixed order absorption (first-order and zero-order absorption rate constants with lag times).
Results
NCA demonstrated comparable maximum plasma concentration (C max) and AUC between age groups for tramadol enantiomers, but significant differences in V area/ F (mean 34 % higher) and k el (mean 28 % lower) in the elderly. PK of ODM were significantly different in the elderly for AUC0–inf (mean 35 % higher), Clr0–48 (mean 29 % lower), and k el (mean 33 % lower). The population analysis identified age as a covariate of V/F (young 305 L; elderly 426 L), with a 50 % longer mean elimination half-life in the elderly. No differences in absorption processes were observed.
Conclusions
Tramadol exposure was similar between the age groups; exposure to ODM was higher in elderly subjects.





Similar content being viewed by others
References
Pergolizzi J, Boger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313.
Jy C. Geriatric clinical pharmacology and clinical trials in the elderly. Transl Clin Pharmacol. 2014;22(2):64–9.
Chien JY, Ho RJ. Drug delivery trends in clinical trials and translational medicine: evaluation of pharmacokinetic properties in special populations. J Pharm Sci. 2011;100(1):53–8.
WHO. Better palliative care for older people. Coppenhagen: WHO Regional Office for Europe; 2004.
Lee CR, McTavish D, Sorkin EM. Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 1993;46(2):313–40.
Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923.
Pascual ML, Fleming RR, Gana TJ, Vorsanger GJ. Open-label study of the safety and effectiveness of long-term therapy with extended-release tramadol in the management of chronic nonmalignant pain. Curr Med Res Opin. 2007;23(10):2531–42.
Fishman RL, Kistler CJ, Ellerbusch MT, Aparicio RT, Swami SS, Shirley ME, et al. Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once-daily tramadol (Tramadol Contramid OAD). J Opioid Manag. 2007;3(5):273–80.
Vorsanger GJ, Xiang J, Gana TJ, Pascual ML, Fleming RR. Extended-release tramadol (tramadol ER) in the treatment of chronic low back pain. J Opioid Manag. 2008;4(2):87–97.
Beaulieu AD, Peloso P, Bensen W, Clark AJ, Watson CP, Gardner-Nix J, et al. A randomized, double-blind, 8-week crossover study of once-daily controlled-release tramadol versus immediate-release tramadol taken as needed for chronic noncancer pain. Clin Ther. 2007;29(1):49–60.
Ummandi S SB, Raghavendra Rao NG, Srikanth Reddy M, Sanjeev Nayak B. Overview on controlled release dosage form. Int J Pharm Sci. 2013;3(4):258–69.
Mongin G. Tramadol extended-release formulations in the management of pain due to osteoarthritis. Expert Rev Neurother. 2007;7(12):1775–84.
Frink MC HH, Englberger W et al. Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittel-Forschung. 1996;46(11):1029–36.
Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988;38(7):877–80.
Paar WD, Poche S, Gerloff J, Dengler HJ. Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur J Clin Pharmacol. 1997;53(3–4):235–9.
Garcia Quetglas E, Azanza JR, Cardenas E, Sadaba B, Campanero MA. Stereoselective pharmacokinetic analysis of tramadol and its main phase I metabolites in healthy subjects after intravenous and oral administration of racemic tramadol. Biopharm Drug Dispos. 2007;28(1):19–33.
Jannetto PJ, Bratanow NC. Utilization of pharmacogenomics and therapeutic drug monitoring for opioid pain management. Pharmacogenomics. 2009;10(7):1157–67.
Lintz W, Erlacin S, Frankus E, Uragg H. Biotransformation of tramadol in man and animal (author’s transl). Arzneimittelforschung. 1981;31(11):1932–43.
Sadean MR, Glass PS. Pharmacokinetics in the elderly. Best Pract Res. 2003;17(2):191–205.
Huang AR, Mallet L. Prescribing opioids in older people. Maturitas. 2013;74(2):123–9.
Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759–65.
Karhu D, Groenewoud G, Potgieter MA, Mould DR. Dose proportionality of once-daily trazodone extended-release caplets under fasting conditions. J Clin Pharmacol. 2010;50(12):1438–49.
Hernandez-Lopez C, Martinez-Farnos L, Karhu D, Perez-Campos T, Rovira S, Encina G. Comparative bioavailability between two tramadol once-daily oral formulations. Methods Find Exp Clin Pharmacol. 2006;28(6):373–8.
Karhu D, El-Jammal A, Dupain T, Gaulin D, Bouchard S. Pharmacokinetics and dose proportionality of three tramadol contramid OAD tablet strengths. Biopharm Drug Dispos. 2007;28(6):323–30.
Karhu D, Fradette C, Potgieter MA, Ferreira MM, Terblanche J. Comparative pharmacokinetics of a once-daily tramadol extended-release tablet and an immediate-release reference product following single-dose and multiple-dose administration. J Clin Pharmacol. 2010;50(5):544–53.
Karhu DBS. Pharmacokinetic evaluation of a novel once-a-day tramadol hydrochloride formulation. Rockville: American College of Clinical Pharmacology Annual Meeting; 2005.
Research FDACfDE. Guidance for industry: Bioanalytical method validation. Maryland: FDA; 2001.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Bonate PL. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer; 2006. p. 387 (xii).
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Efron B. Bayesian inference and the parametric bootstrap. Ann Appl Stat. 2012;6(4):1971–97.
Murthy BP, Skee DM, Danyluk AP, Brett V, Vorsanger GJ, Moskovitz BL. Pharmacokinetic model and simulations of dose conversion from immediate- to extended-release tramadol. Curr Med Res Opin. 2007;23(2):275–84.
Grenier JLJ, Karhu D, editors. Tramadol: from immediate to extended release formulation. In: American College of Clinical Pharmacology 38th Annual Meeting: San Antonio; 12–15 Sept 2009.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Likar R, Wittels M, Molnar M, Kager I, Ziervogel G, Sittl R. Pharmacokinetic and pharmacodynamic properties of tramadol IR and SR in elderly patients: a prospective, age-group-controlled study. Clin Ther. 2006;28(12):2022–39.
Lintz W, Barth H, Osterloh G, Schmidt-Bothelt E. Bioavailability of enteral tramadol formulations. 1st communication: capsules. Arzneimittelforschung. 1986;36(8):1278–83.
Kanaan M, Daali Y, Dayer P, Desmeules J. Uptake/efflux transport of tramadol enantiomers and O-desmethyl-tramadol: focus on P-glycoprotein. Basic Clin Pharmacol Toxicol. 2009;105(3):199–206.
Tzvetkov MV, Saadatmand AR, Lotsch J, Tegeder I, Stingl JC, Brockmoller J. Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin Pharmacol Ther. 2011;90(1):143–50.
Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50(4):1227–40.
Garrido MJ, Habre W, Rombout F, Troconiz IF. Population pharmacokinetic/pharmacodynamic modelling of the analgesic effects of tramadol in pediatrics. Pharm Res. 2006;23(9):2014–23.
Imasogie NN, Singh S, Watson JT, Hurley D, Morley-Forster P. Ultra low-dose naloxone and tramadol/acetaminophen in elderly patients undergoing joint replacement surgery: a pilot study. Pain Res Manag. 2009;14(2):103–8.
Valle M, Garrido MJ, Pavon JM, Calvo R, Troconiz IF. Pharmacokinetic-pharmacodynamic modeling of the antinociceptive effects of main active metabolites of tramadol, (+)-O-desmethyltramadol and (-)-O-desmethyltramadol in rats. J Pharmacol Exp Ther. 2000;293(2):646–53.
Acknowledgments
The study was contracted by Labopharm Inc. MDS Pharma conducted the clinical portions of the study. Dr. Kenneth Swart of Farmovs-Parexel, Bloemfontein, Orange Free State, South Africa, led the quantitative bioanalysis. The authors performed the population PK analysis independently. Dr. Jun Li is acknowledged for his early contribution to model development and preliminary data analysis.
Author contributions
Sybil Skinner-Robertson made substantial contribution to study conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
Caroline Fradette contributed to study conception and design, acquisition of data, manuscript revision, and final approval of the version to be published.
Sylvie Bouchard contributed to study conception and design, manuscript revision, and final approval of the version to be published.
Mohamed Samer Mouksassi contributed to population pharmacokinetic model development, critical review of the manuscript, and final approval of the version to be published.
France Varin made substantial contribution to study conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sybil Skinner-Robertson and Caroline Fradette were employees of Labopharm Inc. prior to Nov 2011. Sylvie Bouchard, France Varin and Mohamad-Samer Mouksassi have no conflicts of interest.
Funding
Labopharm sponsored the conduct of the study. The analyses presented herein were conducted independently as part of the doctoral research of Sybil Skinner-Robertson.
Ethical approval and informed consent
Before initiation of the study, the protocol and informed consent for this study were reviewed and approved by two independent ethics committees (Comité d’Ethique de la Recherche des Sciences de la Santé, Université de Montréal; and Investigational Review Board, MDS Pharma Services, Montreal, QC, Canada). All subjects provided their written informed consent prior to the initiation of any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki as well as the Enoncé de politique des trois Conseils. The study is registered at clinicaltrials.gov (NCT02329561).
Rights and permissions
About this article
Cite this article
Skinner-Robertson, S., Fradette, C., Bouchard, S. et al. Pharmacokinetics of Tramadol and O-Desmethyltramadol Enantiomers Following Administration of Extended-Release Tablets to Elderly and Young Subjects. Drugs Aging 32, 1029–1043 (2015). https://doi.org/10.1007/s40266-015-0315-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-015-0315-4