Abstract
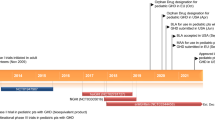
Trofinetide (DAYBUE™), an oral, small molecule, synthetic analog of glycine-proline-glutamate [GPE; the N-terminal tripeptide derivative of insulin like growth factor-1 (IGF-1)], is being developed by Neuren Pharmaceuticals and Acadia Pharmaceuticals for the treatment of rare childhood neurodevelopmental disorders. Trofinetide was approved in March 2023 in the USA for the treatment of Rett syndrome in adult and pediatric patients 2 years of age and older. This article summarizes the milestones in the development of trofinetide leading to this first approval for Rett syndrome.
Similar content being viewed by others
References
Collins BE, Neul JL. Rett syndrome and MECP2 duplication syndrome: disorders of MeCP2 dosage. Neuropsychiatr Dis Treat. 2022;18:2813–35.
Fu C, Armstrong D, Marsh E, et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1): e000717.
Kyle SM, Vashi N, Justice MJ. Rett syndrome: a neurological disorder with metabolic components. Open Biol. 2018;8(2): 170216.
Neul JL, Percy AK, Benke TA, et al. Design and outcome measures of LAVENDER, a phase 3 study of trofinetide for Rett syndrome. Contemp Clin Trials. 2022. https://doi.org/10.1016/j.cct.2022.106704.
Neuren Pharmaceuticals Ltd. Annual Report 2021; 2022. https://www.neurenpharma.com/. Accessed 28 Mar 2023.
Panayotis N, Ehinger Y, Felix MS, et al. State-of-the-art therapies for Rett syndrome. Dev Med Child Neurol. 2023;65(2):162–70.
Silva-Reis SC, Sampaio-Dias IE, Costa VM, et al. Concise overview of glypromate neuropeptide research: from chemistry to pharmacological applications in neurosciences. ACS Chem Neurosci. 2023;14(4):554–72.
US FDA. FDA approves first treatment for Rett syndrome [media release]; 13 Mar 2023. https://www.fda.gov/.
Acadia Pharmaceuticals Inc. Trofinetide: US prescribing information; 2023. https://daybue.com/. Accessed 14 Mar 2023.
Neuren Pharmaceuticals Ltd. FDA approval of DAYBUE™ (trofinetide)–the first approved treatment for Rett syndrome [media release]; 13 Mar 2023. https://www.neurenpharma.com/.
Neuren Pharmaceuticals Ltd. Neuren pipeline; 2023. https://www.neurenpharma.com/. Accessed 29 Mar 2023.
Glaze DG, Neul JL, Percy A, et al. A double-blind, randomized, placebo-controlled clinical study of trofinetide in the treatment of Rett syndrome. Pediatr Neurol. 2017;76:37–46.
Glaze DG, Neul JL, Kaufmann WE, et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92(16):e1912–25.
Berry-Kravis E, Horrigan JP, Tartaglia N, et al. A double-blind, randomized, placebo-controlled clinical study of trofinetide in the treatment of Fragile X syndrome. Pediatr Neurol. 2020;110:30–41.
Acadia Pharmaceuticals Inc, Neuren Pharmaceuticals Ltd. Acadia Pharmaceuticals and Neuren Pharmaceuticals announce exclusive license agreement for the North American development and commercialization of trofinetide in Rett Syndrome [media release]; 6 Aug 2018. https://acadia.com/.
Acadia Pharmaceuticals Inc. Form 10-K; 2022. https://ir.acadia.com/. Accessed 28 Mar 2023.
Bickerdike MJ, Thomas GB, Batchelor DC, et al. NNZ-2566: a Gly-Pro-Glu analogue with neuroprotective efficacy in a rat model of acute focal stroke. J Neurol Sci. 2009;278(1–2):85–90.
Tropea D, Giacometti E, Wilson NR, et al. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106(6):2029–34.
Deacon RM, Glass L, Snape M, et al. NNZ-2566, a novel analog of (1–3) IGF-1, as a potential therapeutic agent for Fragile X syndrome. Neuromolecular Med. 2015;17(1):71–82.
Darwish M, Harlick J, Youakim J, et al. A phase 1, ascending dose study to assess the potential effects of trofinetide on QTc interval, safety and tolerability, and pharmacokinetics in healthy adults [abstract no. 212]. Ann Neurol. 2022;92(Suppl 28):S174.
Darwish M, Youakim JM, Harlick J, et al. A phase 1, open-label study to evaluate the effects of food and evening dosing on the pharmacokinetics of oral trofinetide in healthy adult subjects. Clin Drug Investig. 2022;42(6):513–24.
Percy A, Berry-Kravis E, Lieberman D, et al. Trofinetide for the treatment of Rett syndrome: an open-label study in girls 2 to 4 years of age [abstract no. P13.9-005]. In: American Academy of Neurology (AAN) Annual Meeting; 2023.
Neul J, Percy A, Benke T, et al. Efficacy and safety of trofinetide for the treatment of females with Rett syndrome: results from the randomized, double-blind, phase 3 LAVENDER study [abstract no. 191]. Ann Neurol. 2022;92(Suppl 28):S162–3.
Acadia Pharmaceuticals Inc. 2023 (Data on file).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Trofinetide: First Approval. Drugs 83, 819–824 (2023). https://doi.org/10.1007/s40265-023-01883-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01883-8