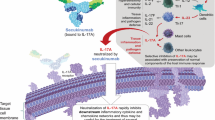
Abstract
Secukinumab (Cosentyx®) is a high affinity, human monoclonal antibody targeted against interleukin (IL)-17A. It is the first-in-class anti-IL-17 agent, initially approved for the treatment of plaque psoriasis, and more recently for the treatment of ankylosing spondylitis and psoriatic arthritis. This article reviews the therapeutic efficacy of subcutaneous secukinumab in the treatment of psoriatic arthritis, as well as discussing the tolerability and pharmacological properties of the drug. Phase III clinical trial data demonstrated that, compared with placebo, subcutaneous secukinumab was efficacious in improving the signs and symptoms of psoriatic arthritis in patients with active disease despite previous treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or disease-modifying anti-rheumatic drugs (DMARDs). In addition, secukinumab treatment was associated with significant improvements in patient-reported measures of physical functioning and health-related quality of life. Secukinumab is generally well tolerated, with the most common adverse events being mild to moderate, non-serious infections, such as upper respiratory tract infections and nasopharyngitis. In conclusion, although longer-term and comparative data are lacking, current clinical data indicate that secukinumab is efficacious and well tolerated in the treatment of psoriatic arthritis, and it thus provides a useful treatment alternative to tumour necrosis factor inhibitors and other targeted DMARDs.

Similar content being viewed by others
References
Anandarajah AP, Ritchlin CT. The diagnosis and treatment of early psoriatic arthritis. Nat Rev Rheumatol. 2009;5(11):634–41.
Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–41.
Mease PJ. Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis. 2011;70(Suppl 1):i77–84.
Ciocon DH, Kimball AB. Psoriasis and psoriatic arthritis: separate or one and the same? Br J Dermatol. 2007;157(5):850–60.
Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology. 2015;54(1):20–8.
Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7.
Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–71.
Olivieri I, D’Angelo S, Palazzi C, et al. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol. 2014;10(9):531–42.
Mease P. A short history of biological therapy for psoriatic arthritis. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S104–8.
Ramiro S, Smolen JS, Landewé R, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2016;75(3):490–8.
Patel DD, Lee DM, Kolbinger F, et al. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii116–23.
Jandus C, Bioley G, Rivals J-P, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307–17.
Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–42.
Benham H, Norris P, Goodall J, et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther. 2013;15(5):R136.
Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130(5):1373–83.
Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17 + CD8 + T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014;66(5):1272–81.
van Baarsen LGM, Lebre MC, van der Coelen D, et al. IL-17 levels in synovium of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis: target validation in various forms of arthritis [abstract no. A184]. Ann Rheum Dis. 2011;70(Suppl 2):A79.
van Baarsen LGM, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16(4):426.
Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359(1–2):419–29.
Noordenbos T, Yeremenko N, Gofita I, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012;64(1):99–109.
European Medicines Agency. Cosentyx: summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 02 Jun 2016.
US FDA. Cosentyx® (secukinumab) injection: US prescribing information. 2016. http://www.fda.gov. Accessed 02 Jun 2016.
Garnock-Jones KP. Secukinumab: a review in moderate to severe plaque psoriasis. Am J Clin Dermatol. 2015;16(4):323–30.
Blair HA, Dhillon S. Secukinumab: a review in ankylosing spondylitis. Drugs. 2016. doi:10.1007/s40265-016-0598-8.
Kolbinger F, Bruin G, Valentin MA, et al. Treatment with secukinumab rapidly leads to positive proteomic and transcriptional changes in psoriatic skin [abstract no. P073]. Exp Dermatol. 2014;23(Suppl 2):11.
Bruin G, Dragatin C, Aigner B, et al. The anti IL-17A IgG1 antibody distributes in dermal interstitial fluid as demonstrated by using dermal open flow microperfusion after a single 300 mg subcutaneous administration; assessment in healthy volunteers and patients with moderate-to-severe plaque psoriasis [abstract no. 106]. J Invest Dermatol. 2014;134(Suppl 1):S18.
Reich K, Papp KA, Matheson RT, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015;24(7):529–35.
Krueger J, Wharton K, Fuentes-Duculan J, et al. Secukinumab reverses disease-defining psoriasis histopathology while retaining full T-cell activation potential [abstract no. P43]. Br J Dermatol. 2014;171(6):e140.
Strober B, Guettner A, Sigurgeirsson B, et al. Secukinumab reduces C-reactive protein levels in patients with moderate to severe plaque psoriasis: result of a regimen-finding study [abstract no. P6395]. J Am Acad Dermatol. 2013;68(4 Suppl 1):AB212.
Gottlieb AB, Langley RG, Philipp S, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol. 2015;14(8):821–33.
Gottlieb AB, Mease P, McInnes IB, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, significantly reduces psoriasis burden in patients with psoriatic arthritis: results from a phase 3 randomized controlled trial [abstract no. 537]. Arthritis Rheumatol. 2014;66(Suppl 10):S233.
McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73(2):349–56.
Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48.
Jansen PAM, Rodijk-Olthuis D, Hollox EJ, et al. β-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One. 2009;4(3):e4725.
Mrowietz U, Qureshi A, Escrig C, et al. Secukinumab treatment shows a neutral effect on the lipid profile of patients with moderate to severe plaque psoriasis: results from a randomized, double-blind, placebo-controlled, phase II study [abstract no. P6871]. J Am Acad Dermatol. 2013;68(4 Suppl 1):AB212.
Philipp S, Escrig C, Papavassilis C, et al. Secukinumab treatment shows a neutral impact on the lipid profile of patients with moderate to severe plaque psoriasis in a dose-ranging study [abstract no. P6508]. J Am Acad Dermatol. 2013;68(4 Suppl 1):AB213.
Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19(10):1597–602.
European Medicines Agency. Assessment report: Cosentyx (secukinumab). 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/003729/WC500199573.pdf. Accessed 20 May 2016.
Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–39.
McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–46.
Ward MM, Guthrie LC, Alba MI. Clinically important changes in individual and composite measures of rheumatoid arthritis activity: thresholds applicable in clinical trials. Ann Rheum Dis. 2015;74(9):1691–6.
Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 2010;70(2):121–45.
Mease PJ, Woolley JM, Bitman B, et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol. 2011;38(11):2461–5.
Mease P, McInnes IB. Secukinumab: a new treatment option for psoriatic arthritis. Rheumatol Ther. 2016. doi:10.1007/s40744-016-0031-5.
van der Heijde D, Landewé RB, Mease PJ, et al. Secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol. 2016. doi:10.1002/art.39685.
Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab provides sustained improvements in psoriatic arthritis: 2-year efficacy and safety results from a phase 3 randomized, double-blind, placebo-controlled trial [abstract no. 2148]. Arthritis Rheumatol. 2015;67(Suppl 10):2576–8.
Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–9.
Gottlieb AB, Thaci D, Blauvelt A, et al. Secukinumab improves skin symptoms and physical functioning compared with ustekinumab in patients with moderate to severe psoriasis with concomitant psoriatic arthritis: subanalysis of a randomized, double blind, parallel-group, active comparator-controlled phase 3b trial [abstract no. 2853]. Arthritis Rheumatol. 2015;67(Suppl 10):3433–4.
Mease P, McInnes IB, Richards H, et al. Secukinumab safety and tolerability in patients with active psoriatic arthritis: pooled safety analysis of two phase 3, randomized, controlled trials (FUTURE 1 and FUTURE 2) [abstract no. SAT0579]. Ann Rheum Dis. 2015;74(Suppl 2):870–1.
Gossec L, Smolen JS. Treatment of psoriatic arthritis: management recommendations. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S73–7.
Acknowledgments
During the peer review process, the manufacturer of secukinumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Matt Shirley and Lesley Scott are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: S. D’Angelo, Rheumatology Department of Lucania, San Carlo Hospital of Potenza, Potenza, Italy; L. R. Espinoza, Section of Rheumatology, Louisiana State University Health Sciences Center, New Orleans, LA, USA; G. Han, Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Rights and permissions
About this article
Cite this article
Shirley, M., Scott, L.J. Secukinumab: A Review in Psoriatic Arthritis. Drugs 76, 1135–1145 (2016). https://doi.org/10.1007/s40265-016-0602-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0602-3