Abstract
Introduction
Bivalent mRNA coronavirus disease 2019 (COVID-19) vaccines may be simultaneously administered with other recommended vaccines, including seasonal influenza vaccines. However, few studies have evaluated the safety of co-administration of bivalent mRNA COVID-19 and seasonal influenza vaccines.
Objective
The aim was to describe reports to the Vaccine Adverse Event Reporting System (VAERS) after co-administration of bivalent mRNA COVID-19 and seasonal influenza vaccines.
Methods
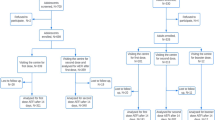
We searched the VAERS database for reports of adverse events (AEs) following co-administration of bivalent mRNA COVID-19 and seasonal influenza vaccines during the period of September 1, 2022–March 31, 2023. We assessed the characteristics of these reports and described the most frequently reported AEs. Clinicians reviewed available medical records for reports of serious AEs and adverse events of special interest (AESI).
Results
During the period of 1 September 2022 through 31 March 2023, VAERS received 3689 reports of AEs following co-administration of bivalent mRNA COVID-19 and seasonal influenza vaccines. The median age of vaccinees was 59 years (interquartile range 39, 70 years); 342 reports (9.3%) were classified as serious. The most common AEs among non-serious reports were severe-acute-respiratory-syndrome-related coronavirus (SARS-CoV-2) infection (785, 23.5%), cough (592, 17.7%), and fatigue (568, 17.0%). The most common AEs among serious reports were Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection (88, 25.7%), dyspnea (81, 23.7%), and condition aggravated (55, 16.1%).
Discussion
Reports of AEs following co-administration of bivalent mRNA COVID-19 and seasonal influenza vaccines did not reveal any unusual or unexpected patterns of AEs. Increased reporting of certain events (e.g., COVID-19) was expected due to Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) reporting requirements. CDC and FDA will continue to monitor the safety of co-administration of mRNA COVID-19 and seasonal influenza vaccines.
Similar content being viewed by others
References
Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. FDA news release. August 31, 2022 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use. Accessed 10 Oct 2022.
CDC expands updated COVID-19 vaccines to include children ages 5 through 11. https://www.cdc.gov/media/releases/2022/s1012-COVID-19-Vaccines.html. Accessed 10 Oct 2023.
Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use. Accessed 10 Oct 2023.
Hause AM, Marquez P, Zhang B, et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among persons aged ≥12 years—United States, August 31–October 23, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1401–6. https://doi.org/10.15585/mmwr.mm7144a3.
Link-Gelles R, Ciesla AA, Fleming-Dutra KE, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection—increasing community access to testing program, United States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1526–30. https://doi.org/10.15585/mmwr.mm7148e1.
Seasonal Influenza Vaccination Resources for Health Professionals. https://www.cdc.gov/flu/professionals/vaccination/index.htm. Accessed 10 Oct 2023.
CDC. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. https://www.cdc.gov/vaccines/COVID-19/clinical-considerations/interim-considerations-us.html#:~:text=COVID%2D19%20vaccination%20is%20recommended,younger%20than%20age%206%20months. Accessed 10 Oct 2023.
Hause AM, Zhang B, Yue X, et al. Reactogenicity of simultaneous COVID-19 mRNA booster and influenza vaccination in the US. JAMA Netw Open. 2022;5(7): e2222241. https://doi.org/10.1001/jamanetworkopen.2022.22241.
Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405. https://doi.org/10.1016/j.vaccine.2015.07.035.
Medical dictionary for regulatory activities. https://www.meddra.org/. Accessed 10 Oct 2023.
Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the Vaccine Adverse Event Reporting System, (VAERS) United States, 2000–2013. Vaccine. 2015;33(28):3171–8. https://doi.org/10.1016/j.vaccine.2015.05.006. (Epub 2015 May 14).
CFR Part 600.80. Postmarketing reporting of adverse experiences. Fed Regist. 1997;7:52252–53.
Rüggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–84. https://doi.org/10.1016/j.vaccine.2007.02.064. (Epub 2007 Mar 12).
Rath B, Gidudu JF, Anyoti H, et al. Facial nerve palsy including Bell’s palsy: case definitions and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. 2017;35(15):1972–83. https://doi.org/10.1016/j.vaccine.2016.05.023. (Epub 2016 May 24).
Sejvar JJ, Kohl KS, Gidudu J, et al. Brighton Collaboration GBS Working Group Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. https://doi.org/10.1016/j.vaccine.2010.06.003.
Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–40. https://doi.org/10.1001/jama.2021.24110.
Centers for Disease Control and Prevention.COVID-19 vaccine safety updates. Advisory Committee on Immunization Practices February 24, 2023 meeting https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-24/COVID-02-Shimabukuro-508.pdf. Accessed 10 Oct 2023.
Murphy SL, Kochanek KD, Xu JQ, Arias E. Mortality in the United States, 2020. NCHS Data Brief, no 427. Hyattsville: National Center for Health Statistics. 2021. https://doi.org/10.15620/cdc:112079. Accessed 10 Oct 2023.
CDC & FDA Identify Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/bivalent-boosters.html. Accessed 10 Oct 2023.
McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32(42):5390–8. https://doi.org/10.1016/j.vaccine.2014.07.073. (Epub 2014 Aug 6).
Woo EJ, Mba-Jonas A, Dimova RB, et al. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA. 2021;326(16):1606–13. https://doi.org/10.1001/jama.2021.16496.
See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56. https://doi.org/10.1001/jama.2021.7517.
Moro PL, Zhang B, Ennulat C, et al. Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the Vaccine Adverse Event Reporting System (VAERS) during July 1, 2021–June 30, 2022. Vaccine. 2023;41(11):1859–63. https://doi.org/10.1016/j.vaccine.2022.12.069. (Epub 2023 Jan 9).
Acknowledgements
We thank the staff of the Immunization Safety Office, and General Dynamics Information Technology, for their work and dedication to public health during the COVID-19 pandemic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding from any organization, agency, or entity was received for this study.
Conflict of interest
None of the authors have conflicts of interest that are directly relevant to the content of this article.
Ethics approval and consent to participate
This activity was reviewed by the CDC and was considered to be consistent with applicable federal law and CDC policy. Informed consent was not obtained for this secondary use of existing information; see 45 CFR part 46.102(l)(2), 21 CFR part 56, 42 USC §241(d), 5 USC §552a, and 44 USC §3501 et seq.
Consent for publication
Not applicable.
Availability of data and materials
Data from the VAERS system is available to everyone through the VAERS Wonder database available at https://wonder.cdc.gov/vaers.html. Anyone with internet access may reach this site and conduct basic analysis to confirm some of the findings described in the article. However, there are data in this paper that come from review of medical records of the patients who experienced an AE. These data cannot be shared openly, to protect patient privacy
Code availability
Not applicable.
Author contributions
PLM originated the study, supervised its implementation, conducted the analysis, and led the writing of the manuscript summarizing the findings. BZ, CE, HB, GW, PM, EJW, and JRS assisted in one or more aspects, including study design, review of VAERS reports and medical records, technical advice, administrative support, and writing of the report. All author read and approved the final version.
Declarations
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the US Food and Drug Administration (FDA). Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC or FDA.
Rights and permissions
About this article
Cite this article
Moro, P.L., Ennulat, C., Brown, H. et al. Safety of Simultaneous Administration of Bivalent mRNA COVID-19 and Influenza Vaccines in the Vaccine Adverse Event Reporting System (VAERS). Drug Saf 47, 487–493 (2024). https://doi.org/10.1007/s40264-024-01406-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-024-01406-8