Abstract
Introduction
Nearly 90% of drugs dispensed in the US are generic products.
Objective
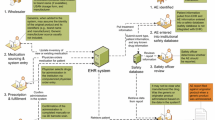
The aim of this study was to develop and implement a tool for analyzing manufacturer-level drug utilization and switching patterns within the US Food and Drug Administration’s Sentinel system.
Methods
A descriptive tool was designed to analyze data in the Sentinel common data model and was tested with two case studies—metoprolol extended release (ER) and lamotrigine ER—using claims data from four Sentinel data partners. We plotted initiators of each brand and generic product over time. For metoprolol ER, we evaluated rates of switching from generics around the time of manufacturing issues. For lamotrigine ER, we examined rates of switching back to the brand among those who switched from brand to generic.
Results
We identified 1,651,285 initiators of metoprolol ER products between July 2008 and September 2015. We observed a large decrease in monthly metoprolol ER initiators (from 25,465 in December 2008 to 13,128 in February 2009), corresponding to recalls by generic manufacturers. We observed simultaneous increases in utilization of the authorized generic and brand products. We identified 4266 initiators of lamotrigine ER with an epilepsy diagnosis between January 2012 and September 2015. Among those who switched from brand to generic, the cumulative incidence of switching back was close to 20% at 2 years. Switchback rates were higher for the first available generic products.
Conclusions
This developed tool was able to elucidate novel utilization and switching patterns in two case studies. Such information can be used to support surveillance of generic drugs and biosimilars.






Similar content being viewed by others
References
Association for Accessible Medicines. Generic drug access & savings in the U.S. 2017. Available at: http://accessiblemeds.org/sites/default/files/2017-07/2017-AAM-Access-Savings-Report-2017-web2.pdf. Accessed 27 Oct 2017.
MedWatch: the FDA safety information and adverse event reporting program. Available at: https://www.fda.gov/Safety/MedWatch/default.htm. Accessed 1 Feb 2018.
Shin JW, Chu K, Jung KH, Lee ST, Moon J, Lee SK. Switching between phenytoin generics in patients with epilepsy may lead to increased risk of breakthrough seizure: chart analysis and practice recommendations. Int J Clin Pharmacol Ther. 2014;52:1017–22.
Andermann F, Duh MS, Gosselin A, Paradis PE. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007;48:464–9.
LeLorier J, Duh MS, Paradis PE, et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology. 2008;70(22 Pt 2):2179–86.
Berg M, Welty TE, Gidal BE, et al. Bioequivalence between generic and branded lamotrigine in people with epilepsy: the EQUIGEN randomized clinical trial. JAMA Neurol. 2017;74:919–26.
Privitera MD, Welty TE, Gidal BE, et al. Generic-to-generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial. Lancet Neurol. 2016;15:365–72.
Ting TY, Jiang W, Lionberger R, et al. Generic lamotrigine versus brand-name Lamictal bioequivalence in patients with epilepsy: a field test of the FDA bioequivalence standard. Epilepsia. 2015;56:1415–24.
Gagne JJ, Polinski JM, Jiang W, et al. Outcomes associated with generic drugs approved using product-specific determinations of therapeutic equivalence. Drugs. 2017;77:427–33.
Gagne JJ, Polinski JM, Jiang W, et al. Switch-backs associated with generic drugs approved using product-specific determinations of therapeutic equivalence. Pharmacoepidemiol Drug Saf. 2016;25:944–52.
Hansen RA, Qian J, Berg RL, et al. Comparison of outcomes following a switch from a brand to an authorized versus independent generic drug. Clin Pharmacol Ther. 2018;103:310–7.
Hansen RA, Qian J, Berg R, et al. Comparison of generic-to-brand switchback rates between generic and authorized generic drugs. Pharmacotherapy. 2017;37:429–37.
Desai RJ, Sarpatwari A, Dejene S, et al. Differences in rates of switchbacks after switching from branded to authorized generics and branded to generic drug products: cohort study. BMJ. 2018;361:k1180.
Platt R, Carnahan RM, Brown JS, et al. The U.S. Food and Drug Administration’s Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):1–8.
Sentinel Snapshot of Database Statistics. Available at: https://www.sentinelinitiative.org/sentinel/snapshot-database-statistics. Accessed 1 Dec 2017.
Sentinel Distributed Database and Common Data Model. Available at: https://www.sentinelinitiative.org/sentinel/data/distributed-database-common-data-model. Accessed 27 Oct 2017.
Zhou M, Wang SV, Leonard CE, et al. Sentinel modular program for propensity score-matched cohort analyses: application to glyburide, glipizide, and serious hypoglycemia. Epidemiology. 2017;28:838–46.
Gagne JJ, Han X, Hennessy S, et al. Successful comparison of US food and drug administration Sentinel analysis tools to traditional approaches in quantifying a known drug-adverse event association. Clin Pharmacol Ther. 2016;100:558–64.
Luo J, Seeger JD, Donneyong M, Gagne JJ, Avorn J, Kesselheim AS. Effect of generic competition on atorvastatin prescribing and patients’ out-of-pocket spending. JAMA Intern Med. 2016;176:1317–23.
Kesselheim AS, Bykov K, Gagne JJ, Wang SV, Choudhry NK. Switching generic antiepileptic drug manufacturer not linked to seizures: a case-crossover study. Neurology. 2016;87:1796–801.
Gagne JJ, Avorn J, Shrank WH, Schneeweiss S. Refilling and switching of antiepileptic drugs and seizure-related events. Clin Pharmacol Ther. 2010;88:347–53.
Popovic JR, Dutcher S, Nguyen M, et al. Sentinel methods development project: identify and evaluate manufacturer-level drug utilization and switching patterns in sentinel. Available at: https://www.sentinelinitiative.org/sites/default/files/Methods/Sentinel_Methods_Manufacturer-Level-Drug-Utilization-Switching-Patterns.pdf. Accessed 16 July 2018.
US Food and Drug Administration National Drug Code Directory. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm. Accessed 27 Oct 2017.
US Food and Drug Administration National Drug Code Directory. Available at: https://www.fda.gov/drugs/informationondrugs/ucm142438.htm. Accessed 16 July 2018.
Sobel RE, Bate A, Marshall J, et al. Do FDA label changes work? Assessment of the 2010 class label change for proton pump inhibitors using the Sentinel System’s analytic tools. Pharmacoepidemiol Drug Saf. 2018;27:332–9.
Fife D, Cepeda MS, Baseman A, et al. Medication changes after switching from CONCERTA® brand methylphenidate HCl to a generic long-acting formulation: a retrospective database study. PLoS One. 2018;13:e0193453.
US Food and Drug Administration Warning Letter (08-ATL-13). Available at: https://wayback.archive-it.org/7993/20170112200220/http:/www.fda.gov/ICECI/EnforcementActions/WarningLetters/2008/ucm1048180.htm. Accessed 31 Oct 2017.
US Food and Drug Administration. ETHEX Corporation issues nationwide voluntary recall of products. Available at: https://wayback.archive-it.org/7993/20170406121711/https://www.fda.gov/Safety/Recalls/ArchiveRecalls/2009/ucm128536.htm. Accessed 27 Oct 2017.
Institute of Good Manufacturing Practices India. Wockhardt recalls metoprolol succinate for third time due to dissolution failure. Available at: http://igmpiindia.org/WockhardtrecallsMetoprololSuccinateforthirdtimeduetodissolutionfailure.html. Accessed 31 Oct 2017.
Jackson I. Generic Toprol XL recalls issued following years of complaints. 2014. Available at: https://www.aboutlawsuits.com/generic-toprol-xl-recall-complaints-67011/. Accessed 31 Oct 2017.
Vossler DG, Anderson GD, Bainbridge J. AES position statement on generic substitution of antiepileptic drugs. Epilepsy Curr. 2016;16:209–11.
US Food and Drug Administration: FY 2015 Awarded GDUFA Regulatory Research Contracts and Grants. Available at: http://wayback.archive-it.org/7993/20171102212740/https:/www.fda.gov/downloads/ForIndustry/UserFees/GenericDrugUserFees/UCM469224.pdf. Accessed 16 July 2018.
Liow K, Barkley GL, Pollard JR, Harden CL, Bazil CW, American Academy of Neurology. Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249–50.
DailyMed.com. Label: lamotrigine tablet, film coated, extended release. Packager: Actavis Pharma, Inc. Updated March 30, 2016. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=87beaf22-593d-4170-bb58-332c615ef90d. Accessed 3 Jan 2018.
Acknowledgements
The authors thank the Data Partners who provided data used in the analysis: Aetna (Aetna Informatics, Blue Bell, PA), HealthCore, (HealthCore, Inc. Government & Academic Research, Alexandria, VA), Humana (Humana, Inc., Comprehensive Health Insights, Miramar, FL), and Optum (OptumInsight Life Sciences Inc., Waltham, MA). The authors thank Timothy Glavin and Adee Kennedy for their assistance. The Sentinel program is funded by the US Food and Drug Administration through the Department of Health and Human Services contract number HHSF223201400030I. The views expressed in this paper are those of the authors and are not intended to convey official US Food and Drug Administration policy or guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of funding
The Sentinel program is funded by the US Food and Drug Administration through the Department of Health and Human Services contract number HHSF223201400030I. The views expressed in this paper are those of the authors and are not intended to convey official US Food and Drug Administration policy or guidance.
Conflict of interest
Joshua J. Gagne has received salary support from grants from Novartis Pharmaceuticals Corporation and Eli Lilly and Company to Brigham and Women’s Hospital and is a consultant to Aetion, Inc. and to Optum, Inc., all for unrelated work. Jennifer R. Popovic, Michael Nguyen, Sukhminder K. Sandhu, Patty Greene, Rima Izem, Wenlei Jiang, Zhong Wang, Yueqin Zhao, Andrew B. Petrone, Anita K. Wagner, Sarah K. Dutcher have no conflicts of interest.
Research involving human participants
This study was conducted within the Sentinel system and represents a public health activity under the auspices of the US Food and Drug Administration. Activities conducted within Sentinel are not under the purview of institutional review boards.
Appendix
Appendix
Metoprolol ER products and approval dates | |
|---|---|
Group | Product Approval Date |
ToprolXL_Brand | January 10, 1992 |
Metoprolol_AG_Par | November 21, 2006a |
Metoprolol_Sandoz | July 31, 2006 |
Metoprolol_Nesher | May 18, 2007 |
Metoprolol_Actavis | August 3, 2009 |
Metoprolol_Wockhardt | July 22, 2010 |
Metoprolol_Mylan | December 15, 2011 |
Metoprolol_DrReddys | August 1, 2012 |
Lamotrigine ER products and approval dates | |
|---|---|
Group | Product Approval Date |
LamictalXR_Brand | May 29, 2009 |
Lamotrigine_Anchenb | December 26, 2012 |
Lamotrigine_Wockhardt | January 4, 2013 |
Lamotrigine_Par | January 18, 2013 |
Lamotrigine_Wilshire | June 17, 2013 |
Lamotrigine_DrReddys | June 19, 2013 |
Lamotrigine_Actavis | October 17, 2013 |
Lamotrigine_Torrentb | December 23, 2013 |
Rights and permissions
About this article
Cite this article
Gagne, J.J., Popovic, J.R., Nguyen, M. et al. Evaluation of Switching Patterns in FDA’s Sentinel System: A New Tool to Assess Generic Drugs. Drug Saf 41, 1313–1323 (2018). https://doi.org/10.1007/s40264-018-0709-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0709-4