Abstract
Background and Objective
OnabotulinumtoxinA (BoNTA) is a relatively safe and effective treatment for chronic migraine. The local mode of action of BoNTA favors the combination of oral treatments with systemic action. However, little is known about the possible interactions with other preventive treatments. The objective of the study was to describe the use of oral preventive treatments in patients with chronic migraine treated with BoNTA in routine clinical care and discuss the tolerability and efficacy according to the presence or absence of concomitant oral treatments.
Methods
In this multicenter, observational, retrospective, cohort study, we collected data from patients with chronic migraine receiving prophylactic treatment with BoNTA. Patients were eligible if aged ≥18 years, diagnosed with chronic migraine according to the International Classification of Headache Disorders, Third Edition criteria, and treated with BoNTA according to the PREEMPT paradigm. We documented the proportion of patients with at least one concomitant treatment prescribed specifically for migraine (CT+M) and their side effects during four BoNTA treatment cycles. Additionally, we collected monthly headache days and monthly acute medication days from the patients’ headache diaries. Patients with CT+M were compared to those without concomitant treatment (CT−) using a nonparametric approach.
Results
Our cohort included 181 patients taking BoNTA, of whom 77 (42.5%) received a CT+M. The most frequently prescribed concomitant treatments were antidepressants and antihypertensive drugs. Side effects in the CT+M group occurred in 14 patients (18.2%). Only in three of them (3.9%), the side effects had a significant interference with the patient’s functioning (all in topiramate 200-mg/day users). Both CT+M and CT− groups had a significant reduction in monthly headache days of respectively − 6 (95% confidence interval − 9, − 3; p < 0.001; w = 0.200) during cycle 4 compared with baseline versus − 9 (95% confidence interval − 13, −6; p < 0.001; w = 0.469). However, the reduction in monthly headache days was significantly smaller in patients with CT+M after the fourth treatment cycle compared with patients with CT− (p = 0.004).
Conclusions
Prescription of oral concomitant preventive treatment is common in patients with chronic migraine receiving BoNTA. We did not identify any unexpected safety or tolerability issues in patients receiving BoNTA and a CT+M. However, patients with a CT+M experienced a smaller reduction in monthly headache days when compared with those with CT−, which might be associated with a higher resistance to treatment in that subgroup of patients.
Similar content being viewed by others
In clinical practice, oral concomitant treatments are common in patients with chronic migraine treated with onabotulinumtoxinA. |
Patients with oral concurrent migraine treatment showed a lower reduction in the number of monthly headache days, which may be explained by potential higher treatment resistance. |
The use of oral concomitant treatments in patients with chronic migraine treated with onabotulinumtoxinA did not lead to unexpected safety concerns. |
1 Introduction
Migraine is a complex and multifactorial neurological disease often requiring preventive treatment [1]. OnabotulinumtoxinA (BoNTA) is the first treatment specifically approved for the prevention of chronic migraine (CM) [2]. For this indication, BoNTA is injected in multiple pericranial muscles, at a total dosage of 155–195 U, every 12 weeks [3]. This paradigm of BoNTA injection has proven safe and effective for the prevention of CM in the PREEMPT trials [4,5,6] and its effectiveness has been confirmed in several real-world studies [7,8,9,10,11,12,13,14,15,16]. In these studies, the percentage of patients with a ≥ 50% reduction in monthly headache days varied from 47 to 69% [17]. This represents a very favorable efficacy outcome in the context of the available migraine preventive treatments [18].
Despite these positive findings, in some patients, the reduction in headache days with BoNTA alone is not satisfactory. The European Headache Federation states that combinations of preventive treatments should be considered in patients with unsatisfactory responses to treatment [19].
The local mode of action of BoNTA may favor the combination with concomitant treatments (CT). In the PREEMPT trial population, the use of CT together with BoNTA was prohibited [4,5,6]. Conversely, in clinical practice, the use of CT is very frequent [20]. Recently, BoNTA has been used in combination with monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway with positive results [21,22,23,24,25,26].
Despite the feasibility and frequent use of CT in combination with BoNTA in clinical practice, to date, very few studies have focused on the impact of CT on migraine outcomes of patients treated with BoNTA. A Spanish multicenter study found that 90% of patients with CM treated with BoNTA used CT when the treatment was initiated, and 41% of them were able to withdraw all oral treatments during treatment with BoNTA [20]. However, no detail was provided on the pattern of use of CT, concerning the start of therapy, withdrawal, and dose change. Reporting details on the use of treatments in combination with BoNTA would provide useful guidance in routine clinical practice, especially in terms of drug interactions and therapeutic synergies.
We, therefore, performed an international, multicenter, real-world study to report detailed treatment patterns and to test the tolerability and effectiveness of oral CT in patients with CM receiving BoNTA treatment. Our primary objective was to describe concomitant pharmacological treatment patterns in patients treated with BoNTA. Secondary objectives included the comparison of monthly headache days (MHD) and monthly days with acute medication use (AMD) between patients with concomitant oral treatment (CT+) and those without (CT−), and stating side effects associated with the concomitant treatment by use of the Clinical Global Impressions Scale-Efficacy Index [27].
2 Methods
2.1 Study Design and Setting
This is a multicenter, observational, retrospective, cohort study conducted at the Neurology Departments of Charité Universitätsmedizin Berlin, IRCCS Mondino Foundation of Pavia, Italy, and the University of L’Aquila, Italy. We collected data from patients with CM receiving preventive treatment with BoNTA between January 2016 and March 2021. Data from the first four treatment cycles were obtained from all eligible patients.
The study was approved by the ethics committees of the three participating sites under local regulations (Berlin AE1/159/22, Pavia 0097925/21, and L’Aquila 34/2021). Informed consent was obtained from all patients enrolled in Pavia and L’Aquila centers, while the document was not required in Berlin because of the retrospective nature of the study. This study was performed in accordance with the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement for cohort studies.
2.2 Participants and Study Size
2.2.1 Study Groups
This study included two study groups. Group 1 included patients who received only BoNTA treatment without any concomitant oral treatments (CT−). Group 2 included all patients who received BoNTA and at least one concomitant oral treatment with a potential effect on migraine (CT+) during at least one treatment cycle. We considered anticonvulsants, antidepressants, and antihypertensive drugs as drug classes with a potential effect on migraine. Additionally, all drugs prescribed by a headache specialist for the indication of migraine prophylactic treatment were assumed to have a potential effect on migraine.
We stratified group 2 based on the indication of the CT into patients with at least one CT prescribed specifically for migraine (CT+M) and those with CT prescribed not for migraine (CT+O) [e.g., prescribed for hypertension or depression]. The decision to start, modify, or stop a concomitant treatment was based on the clinical judgment of the treating physicians.
2.2.2 Patient Selection
We screened the electronic charts of all patients with migraine who received BoNTA in the selected period. Inclusion criteria for this analysis were: (1) age ≥18 years at treatment initiation, (2) diagnosis of CM according to the International Classification of Headache Disorders, Third Edition criteria [28], and (3) health records and headache diary data availability for at least four subsequent treatment cycles with BoNTA according to the PREEMPT injection paradigm (155–195 U in 31–39 injection sites every 12 weeks) [29]. We excluded patients with insufficient health and/or headache documentation or who had already received BoNTA treatment before the observation period. Because of the retrospective observational nature of the study, we did not calculate a sample size but simply enrolled all eligible patients over the period indicated above.
2.3 Data Sources
All data were obtained from the electronic patient records. We collected general health history and headache-specific history. Currently used medications were classified into with/without a potential migraine preventive effect and prescribed/not prescribed for migraine as mentioned above. Headache-related variables were extracted from electronic or paper headache diaries. A headache day was defined as a day on which at least 30 minutes of headache was reported with a visual analog scale score (range 0–10) of at least 1. An acute medication day was defined as any headache day on which acute medication was taken. Acute medication included nonsteroidal anti-inflammatory drugs, analgesics, and/or triptans.
2.4 Variables
We evaluated demographic variables, including age, sex, migraine disease duration, the occurrence of migraine aura, prior migraine prophylactic treatments before BoNTA treatment, and current medications. From the headache diaries, we extracted the variables: MHD and AMD. For this analysis, a month was defined as a 28-day period. A MHD was defined as a day with a reported headache of at least 30 minutes and an AMD as a day with a headache on which acute medication (non-steroidal anti-inflammatory drug and/or triptan) was used. The baseline MHD and AMD values were calculated from the 4 weeks before BoNTA initiation. The mean MHD and AMD were also calculated for every 12 weeks after each of the four BoNTA treatments. Based on these variables, we calculated the 30% and 50% responder rates, which represent the percentage of patients with a reduction in MHD by at least 30% or 50% from baseline during the 12-week periods after each BoNTA cycle. Based on the electronic charts of the patient’s treatment, we assessed the side effects associated with the CT+M and scored them according to the Clinical Global Impressions Scale-Efficacy Index [27]. The side effects were ranked as “None,” “Do not significantly interfere with patient functioning,” “Significantly interfere with patient functioning,” or “Outweigh the therapeutic effect,” based on the available documentation.
2.5 Statistical Analyses
We reported continuous variables as means and standard deviations or median and 95% confidence interval (CI) for the median. Categorical variables were reported as number (%). In the case of missing headache documentation, we used the last observation carried forward approach. Patients for whom it was not possible to calculate MHD and AMD at baseline and/or from at least two out of the four follow-ups were excluded.
As our data were not normally distributed, as assessed with the Kolmogorov–Smirnov test, we used non-parametric statistics. We used Friedman’s two-way analysis of variance by rank test for repeated measurements with a Dunn’s pairwise post-hoc-test. P-values were adjusted by the Bonferroni correction for multiple testing. To assess the effect size, we estimated Kendall’s Concordance Coefficient W, where 0 (indicates no relationship) and 1 (indicates a perfect relationship). To compare groups, we applied the Independent-Samples Mann–Whitney U Test or the Independent-Samples Kruskal–Wallis Test as appropriate. A value of p < 0.05 was considered statistically significant. Statistical analyses were performed with IBM SPSS Statistics, version 28.0.1.0 (IBM, Armonk, NY, USA).
3 Results
3.1 Participants
At all three centers, 314 patients were treated with at least four cycles of BoNTA between January 2016 and March 2021. Among those, we identified 178 patients who were eligible for our study. Patient disposition and reasons for exclusion are displayed in the flow chart (Fig. 1).
3.2 Demographic Characteristics
Of the 178 included patients, 70 (39.3%) patients only received a BoNTA treatment without any concomitant oral treatments (CT−). The remaining 108 (60.7%) patients received at least one concomitant oral treatment (CT+).
Patients in the CT− group were on average younger compared with patients in the CT+ group (p = 0.005). On average, patients had already not responded to 2.6 ± 1.5 prior preventive treatments, which were discontinued because of a lack of efficacy or limiting side effects.
Almost half (n = 87, 48.9%) of our patients were diagnosed with medication overuse headache. Patients with CT+ and CT− had a comparable incidence of medication overuse headache (49.1% vs 48.9%, respectively). Patients with CT+, however, were more likely to experience a medication overuse headache previously (23.1% vs 5.7%, respectively). A mood disorder was diagnosed in 73 patients (41.0%) and an anxiety disorder in 61 (34.3%). Mood or anxiety disorders and hypertension were diagnosed more often in patients with CT+ compared with patients with CT− (respectively, p = 0.003, p < 0.001, and p = 0.003). All patients’ characteristics are shown in Table 1.
3.3 Concomitant Oral Treatment
3.3.1 Concomitant Migraine Prophylactic Treatment
Our cohort included 74 (41.6%) patients who received at least one concomitant oral treatment prescribed specifically for migraine (CT+M) during the first four treatments with BoNTA. These patients received one (n = 53, 71.6%), two (n = 12, 16.2%), or three (n = 9, 12.2%) CT+M. Table 2 provides an overview of the prescribed CT+M drugs.
From the patients with one CT (n = 53, 71.6%), the CT remained unchanged in 31 patients (58%) during the first four treatment cycles with BoNTA. Eight patients (15%) started CT and 13 (25%) stopped CT. One patient (2%) started and stopped CT over the course of 1 year with BoNTA.
Of the patients with two CT (n = 12, 16.2%), five patients (52%) did not change the CT during the first four cycles with BoNTA. Two patients (17%) stopped one CT, one patient (8%) started one CT, and one patient (8%) started and stopped one CT. Two patients (17%) started and stopped both CTs, and one patient stopped both CTs.
From the patients with three CTs (n = 9, 12.2%), all CTs remained unchanged in two patients (22%). Three patients (33%) started a third CT and one (11%) stopped a third CT. Three patients (33%) started a second and third CT.
Side effects caused by the CT+M during the first four treatment cycles with BoNTA were reported by 15 (20.3%) patients. Only in three (4.1%) of these patients did side effects (all in topiramate 200-mg daily users) significantly interfere with the patient’s functioning according to the Clinical Global Impression-Efficacy Index. Table 1 in the Electronic Supplementary Material (ESM) shows the number of side effects and the corresponding drug per drug class.
3.3.2 Concomitant Oral Medication Not For Headache
34 patients (19.1%) receive a concomitant oral treatment prescribed for an indication other than headache (CT+O). The majority had one additional treatment (n = 21, 61.8%). Thirteen (38.2%) patients had two additional treatments. The CT+O most frequently prescribed were antidepressants in 23 (67.6%) patients and antihypertensive drugs in 16 (47.1%) patients. In the majority of these patients (n = 31, 91.2%), the treatment remained unchanged during the first four cycles with BoNTA. A detailed overview of prescribed treatments not for headache is shown in Table 3.
3.4 Monthly Headache Days
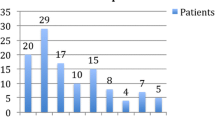
Table 4 shows MHD at baseline and their change after the first four treatment cycles with BoNTA for patients with CT−, CT+, and the strata CT+M and CT+O. In the CT− group (n = 70, 39.3%), the median MHD decreased from 20 (95% CI 19, 25) at baseline to 8.5 (95% CI 7, 10) in the 12 weeks after the fourth treatment cycle with BoNTA (p < 0.001). Patients with CT+ (n = 108, 60.7%) also showed a significant reduction in MHD from 22 (95% CI 20, 25) at baseline to 11 (95% CI 9, 14) during the 12 weeks after the fourth cycle with BoNTA (p < 0.001). Figure 2 illustrates the ≥ 30% and ≥ 50% responder rates in the total population and the CT− and CT+ groups. A reduction of at least 30% of MHD was achieved by 49 (70.0%) and 59 (54.6%) of patients with CT− and CT+, respectively, after cycle 2 compared with baseline (p = 0.043). After cycle 4, a reduction of at least 30% of MHD compared with baseline was achieved by 52 (74.3%) and 63 (58.3%) of patients with CT− and CT+, respectively (p = 0.037). We did not detect significant differences between patients with CT− and CT+ for the ≥ 50% responder rate.
In the stratification for CT+M (n = 74) versus CT+O (n = 34), both groups had a significant reduction in MHD from baseline to the first four treatment cycles with BoNTA (p < 0.001 for both groups). However, patients with a CT+M showed a significantly lower reduction in MHD after cycles 2–4, compared to patients with CT− (p = 0.002, p = 0.001, and p = 0.004, respectively) and to patients with CT+O (p = 0.008, p < 0.001, and p = 0.006 respectively). Figure 3 illustrates the ≥ 30% and ≥ 50% responder rates in the two population strata. After cycle 3, 26 (76.5%) and 24 (70.6%) patients with CT+O achieved, respectively, a 30% and 50% reduction of MHD compared with baseline. For patients with CT+M, these were respectively 37 (50.0%, p = 0.012) and 27 (36.5%, p = 0.002) patients. A 30% and 50% reduction of MHD compared with baseline was achieved by 26 (67.5%) and 21 (61.8%) patients with CT+O compared with 37 (50.0%) and 28 (37.8%) patients with CT+M, respectively (p = 0.012 and p = 0.024).
3.5 Monthly Acute Medication Days
Table 5 shows the change in AMD from baseline and after the first four treatment cycles with BoNTA for patients with CT−, CT+, and the strata CT+M and CT+O. In the total cohort, AMD decreased from a median of 15 (95% CI 15, 17) during baseline to 7 (95% CI 6, 8) after the fourth treatment cycle with BoNTA (p < 0.001). A difference in the reduction of AMD was observed after cycle 2 between CT− and CT+M (p = 0.014) and between CT+M and CT+O (p = 0.019). During cycle 3, a difference between patients with CT+M and CT+O (p = 0.030) was observed.
4 Discussion
In this multicenter real-life study, BoNTA treatment was well tolerated, and no important side effects were registered even with an additional concomitant oral preventive drug. Our data confirmed the well-established efficacy of BoNTA with a median reduction of 8 MHD after the fourth treatment cycle compared with baseline, regardless of the use of a concomitant oral preventive treatment, in line with previous reports [6, 30]. After stratification, we observed a greater reduction in MHD in patients without concomitant oral treatment after cycles 2–4 compared with patients with oral concomitant oral treatments. This observation could probably be explained by a higher disease burden in patients with oral concomitant therapies as suggested by the higher number of prior preventive treatments, and psychological and non-psychological comorbidities. These clinical features are well-known negative predictors of response to migraine prevention therapies [31].
Our cohort highlights the high frequency of polypharmacological treatment patterns in patients with CM. The majority of patients treated with BoNTA (60.7%) were taking concomitant medications. Among these, for 74 (41.6%) patients, these drugs were prescribed with an exclusive indication for migraine, while the remaining had other comorbidities for which they required medications such as anxiety or depression. Polytherapy was well tolerated, and side effects were registered in 15 subjects only (20.3%). That is significantly lower than reported in the literature regarding oral medications, which are well known for lacking persistence [32]. Most patients in our study were already taking a well-tolerated, but not sufficiently effective CT at a stable dose before starting BoNTA treatment, thus minimizing possible collateral effects. However, in some patients, the possible use of a lower-than-average dose of oral treatment (e.g., a low dose of amitriptyline as shown in Table 2, compared to the minimum/standard dose recognized as effective [19]) could lead to a lower-than-expected incidence of adverse events and a higher persistence among the patients.
Monotherapy is recommended for migraine preventive treatment based on the evidence available in the literature and clinical practice [33]. However, patients with CM are notably difficult to treat and often resistant or refractory to treatments [19]. Therefore, they often require polytherapy. For example, up to 63.3% of cases of patients with medication overuse headache were receiving polytherapy when admitted for a detoxification program in a tertiary headache center in Italy [34]. In addition, polytherapy could be justified even in patients who have a good response to migraine treatments, but a high number of residual MHD [35]. However, to date, only topiramate, anti-CGRP(-receptor) antibodies, and BoNTA are the treatments specifically approved for CM prophylaxis and few data regarding the use of concomitant therapies are available from real-life studies (Table 2 in the ESM). In most of these studies, patients receiving BoNTA were also taking concomitant oral medications, but neither efficacy nor safety was assessed by stratifying the population according to this parameter. The COMPEL study found that patients receiving concomitant preventive treatment at baseline had a significantly smaller reduction in MHD from baseline at week 108 in comparison with patients not receiving preventive treatment at baseline [36]. Casucci et al. [37] suggested that add-on therapy should be considered in patients who present with high disability [34], concurrent risk factors or comorbidities, and a history of failed preventive treatments. A recent Italian consensus paper suggests adding concomitant prophylaxis when the ≥ 50% reduction in MHD is not achieved and no improvements in any of the efficacy/benefit indicators are observed [38]. In this framework, our data support the possibility of using concomitant oral prophylaxis in those difficult-to-treat patients or those lacking a sufficient response to BoNTA, with no fear of an increase in adverse event incidence.
In this real-life multicenter study, it is also possible to observe the debatable choice of using off-label drugs that are not recommended by current guidelines specifically for migraine prophylaxis, such as gabapentin among the anticonvulsants or quetiapine, a second-generation antipsychotic. This choice probably derives from a few studies that assessed the effectiveness of the above-mentioned molecules even though there is insufficient evidence to support their inclusion in the list of effective therapies [39, 40]. However, this phenomenon points to the necessity for physicians to find acceptable alternatives to help resistant/refractory patients.
Finally, it would be interesting to address the reasons why many drugs were kept once BoNTA was added to the prophylaxis. Lacking a demonstration of the BoNTA additive effect over the other classes of treatment, this observation could reflect the fear of both patients and physicians that the improvement obtained with BoNTA may be jeopardized by the interruption.
Overall, this study is not without limitations. First, it was a retrospective observational study, although the strength of the data is supported by the use of documented health records and by the prospectively compiled headache diaries. Second, we collected data over a 4-year observation period that coincided with the advent of the CGRP-targeting monoclonal antibody. This event is likely to have introduced dynamic changes in the routine management of subjects. Moreover, the lack of consensus on the minimum/target dosage for oral migraine preventive treatments and on the duration of exposure for the assessment of efficacy may have introduced a huge variability among the different dosing schemes [19], especially when it comes to the use of these molecules for two different purposes (e.g., hypertension and migraine or mood disorder and migraine). As a real-life study, it was decided to include all the possible dosages used to depict the different attitudes toward combination therapy. To create a homogeneous dataset that was as complete as possible, we excluded patients with incomplete data or early treatment termination. Furthermore, we excluded patients with prior experience with BoNTA, as complete information about the effectiveness was not available at our centers and we preferred including “naïve” subjects only. Therefore, only those who started the treatment during the timeframe mentioned were considered for a more comprehensive analysis. This could have led to selection bias, excluding patients with poor response or with compliance issues with BoNTA and/or the other migraine preventive treatments. Moreover, the duration of our baseline (28 days before the first treatment cycle) might be too short for an accurate representative baseline, as the MHD might vary from month to month. Therefore, part of our results may have been caused by the natural course and monthly variance of the headache frequency. However, our sample size is large enough to reduce this bias to a minimum. Finally, we did not consider if CT was started before or after BoNTA treatment in our analysis. Moreover, we did not stratify for this feature in the subsequent comparative analysis. This may introduce bias, which could lead to an underestimation of the true association and the possible synergic effect of the two types of medication.
5 Conclusions
This multicenter retrospective study described the real-life clinical practice regarding the use of combinations of oral preventive medications and BoNTA in a large cohort of patients with a follow-up of at least 12 months. In our cohort of patients treated with BoNTA, concomitant migraine prevention demonstrated excellent tolerability, and we did not observe unexpected safety concerns. Patients treated with a combination of prophylaxis treatment reported a smaller reduction in MHD, possibly because of a higher burden of disease, while we did not detect any signal pointing toward safety concerns when combining oral preventive treatments with BoNTA. Further studies are needed to address the real extent of the additive effect that the combination of different drugs might have in specifically selected populations such as those with resistant or refractory migraine or medication overuse.
References
Ashina M. Migraine. N Engl J Med. 2020;383:1866–76. https://doi.org/10.1056/NEJMra1915327.
Ray JC, Hutton EJ, Matharu M. OnabotulinumtoxinA in migraine: a review of the literature and factors associated with efficacy. J Clin Med. 2021;10(13):2898.
Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50(9):1406–18.
Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803.
Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14.
Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–36.
Kollewe K, Gaul C, Gendolla A, Sommer K. Real-life use of onabotulinumtoxinA reduces healthcare resource utilization in individuals with chronic migraine: the REPOSE study. J Headache Pain. 2021;22(1):50.
Vernieri F, Paolucci M, Altamura C, Pasqualetti P, Mastrangelo V, Pierangeli G, et al. OnabotulinumtoxinA for chronic migraine: a real-life Italian multicenter experience. Neurol Sci. 2018;39(Suppl 1):171–2.
Santoro A, Copetti M, Miscio AM, Leone MA, Fontana A. Chronic migraine long-term regular treatment with onabotulinumtoxinA: a retrospective real-life observational study up to 4 years of therapy. Neurol Sci. 2020;41(7):1809–20.
Alpuente A, Gallardo VJ, Torres-Ferrús M, Álvarez-Sabin J, Pozo-Rosich P. Short and mid-term predictors of response to onabotulinumtoxinA: real-life experience observational study. Headache. 2020;60(4):677–85.
Kollewe K, Escher CM, Wulff DU, Fathi D, Paracka L, Mohammadi B, et al. Long-term treatment of chronic migraine with onabotulinumtoxinA: efficacy, quality of life and tolerability in a real-life setting. J Neural Transm (Vienna). 2016;123(5):533–40.
Beckmann Y, Çetin Üncü F, Kurt İncesu T, Türe S. Effectiveness, safety, and health-related quality of life of chronic migraine patients treated with onabotulinum toxin A. Eur Neurol. 2020;83(5):517–22.
Ornello R, Guerzoni S, Baraldi C, Evangelista L, Frattale I, Marini C, et al. Sustained response to onabotulinumtoxin A in patients with chronic migraine: real-life data. J Headache Pain. 2020;21(1):40.
Aicua-Rapun I, Martínez-Velasco E, Rojo A, Hernando A, Ruiz M, Carreres A, et al. Real-life data in 115 chronic migraine patients treated with onabotulinumtoxin A during more than one year. J Headache Pain. 2016;17(1):112.
Khalil M, Zafar HW, Quarshie V, Ahmed F. Prospective analysis of the use of OnabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, U.K. J Headache Pain. 2014;15(1):54.
Andreou AP, Trimboli M, Al-Kaisy A, Murphy M, Palmisani S, Fenech C, et al. Prospective real-world analysis of onabotulinumtoxinA in chronic migraine post-National Institute for Health and Care Excellence UK technology appraisal. Eur J Neurol. 2018;25(8):1069-e83.
Wang YF. OnabotulinumtoxinA injection in the treatment of chronic migraine. Prog Brain Res. 2020;255:171–206.
Agostoni EC, Barbanti P, Calabresi P, Colombo B, Cortelli P, Frediani F, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20(1):92.
Sacco S, Braschinsky M, Ducros A, Lampl C, Little P, van den Brink AM, et al. European Headache Federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain. 2020;21(1):76.
Alpuente A, Gallardo VJ, Torres-Ferrús M, Santos-Lasaosa S, Guerrero AL, Laínez JM, et al. Evaluation of the concomitant use of oral preventive treatments and onabotulinumtoxinA in chronic migraine: the PREVENBOX study. Eur J Neurol. 2020;27(10):2102–8.
Blumenfeld AM, Frishberg BM, Schim JD, Iannone A, Schneider G, Yedigarova L, et al. Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther. 2021;10(2):809–26.
Guerzoni S, Baraldi C, Pani L. The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: a reliable option for the optimal treatment of chronic migraine. Neurol Sci. 2022;43(9):5687–95.
Armanious M, Khalil N, Lu Y, Jimenez-Sanders R. Erenumab and onabotulinumtoxinA combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J Pain Palliat Care Pharmacother. 2021;35(1):1–6.
Cohen F, Armand C, Lipton RB, Vollbracht S. Efficacy and tolerability of calcitonin gene-related peptide-targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med. 2021;22(8):1857–63.
Mechtler L, Saikali N, McVige J, Hughes O, Traut A, Adams AM. Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to onabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front Neurol. 2021;12: 788159.
Silvestro M, Tessitore A, Scotto di Clemente F, Battista G, Tedeschi G, Russo A. Additive interaction between onabotulinumtoxin-A and erenumab in patients with refractory migraine. Front Neurol. 2021;12:656294.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37.
No authors listed. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Bendtsen L, Sacco S, Ashina M, Mitsikostas D, Ahmed F, Pozo-Rosich P, et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain. 2018;19(1):91.
Aurora SK, Dodick DW, Diener HC, DeGryse RE, Turkel CC, Lipton RB, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014;129(1):61–70.
Schiano di Cola F, Caratozzolo S, Liberini P, Rao R, Padovani A. Response predictors in chronic migraine: medication overuse and depressive symptoms negatively impact onabotulinumtoxin-A treatment. Front Neurol. 2019;10:678.
Hepp Z, Dodick DW, Varon SF, Chia J, Matthew N, Gillard P, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–85.
D’Amico D. Controversies in migraine: monotherapy. Neurol Sci. 2012;33(Suppl 1):S141-5.
D’Amico D, Curone M, Tullo V, Proietti Cecchini A, Mea E, Bussone G. Polytherapy for the prophylaxis of chronic migraine: an Italian survey. Neurol Sci. 2011;32(Suppl 1):S185-8.
Ornello R, Baraldi C, Guerzoni S, Lambru G, Andreou AP, Raffaelli B, et al. Comparing the relative and absolute effect of erenumab: is a 50% response enough? Results from the ESTEEMen study. J Headache Pain. 2022;23(1):38.
Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Manack AA. Long-term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19(1):13.
Casucci G, Villani V, Cologno D, et al. Polytherapy for migraine prophylaxis. Neurol Sci 2012;33(Suppl 1):147–150. https://doi.org/10.1007/s10072-012-1060-7.
Sacco S, Russo A, Geppetti P, Grazzi L, Negro A, Tassorelli C, et al. What is changing in chronic migraine treatment? An algorithm for onabotulinumtoxinA treatment by the Italian chronic migraine group. Expert Rev Neurother. 2020;20(12):1275–86.
Bagnato F, Good J. The use of antiepileptics in migraine prophylaxis. Headache. 2016;56(3):603–15.
Krymchantowski AV, Jevoux C, Moreira PF. An open pilot study assessing the benefits of quetiapine for the prevention of migraine refractory to the combination of atenolol, nortriptyline, and flunarizine. Pain Med. 2010;11(1):48–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest/Competing Interests
Lucas Hendrik Overeem, Maria Magdalena Pocora, and Daniele Martinelli have no conflicts of interest that are directly relevant to the content of this article. Raffaele Ornello has served on advisory boards for Eli Lilly; received lecture honoraria from Novartis, Eli Lilly, and Teva; received research funding from Novartis and Allergan-AbbVie; and serves on the editorial boards of Frontiers in Pain Research, Frontiers in Neurology, The Journal of Headache and Pain, and Brain Sciences. Uwe Reuter has served on advisory boards for Amgen, Allergan, Abbvie, Eli Lilly, Lundbeck, Novartis, and Teva and has received honoraria for lectures from Amgen, Allergan, Abbvie, Eli Lilly, Lundbeck, Novartis, electroCore, Medscape, StreaMedUp, and Teva, received honoraria for consulting services from Lundbeck, Pfizer, and Abbvie; received research funding from Novartis (CHERUB01) and the German Federal Ministry of Education and Research; and is an associate editor of the Journal of Headache and Pain and Frontiers in Neurology. Simona Sacco has received lecture honoraria from Abbott, Allergan-Abbvie, AstraZeneca, Eli Lilly, Lundbeck, Novartis, NovoNordisk, Pfizer, and Teva, has received research funding from Allergan, Novartis, Uriach, Medscape, and Neurodiem Ology Medical Education, and is Editor-in-Chief of Frontiers in Neurology - Headache and Neurogenic Pain, associate editor of the Journal of Headache and Pain, Associate Editor of Stroke, co-chair of the European Stroke Organization Guidelines Committee, and a board member of the European Headache Federation. Cristina Tassorelli has served on the advisory boards of Abbvie, Dompé, Eli-Lilly, Lundbeck, Novartis, and Teva; received lecture honoraria from Abbvie, Eli-Lilly, Lundbeck, Novartis, and Teva; honoraria for consulting services from Medscape; received research funding from the EU Commission, the Italian Ministry of Health, the American Migraine Foundation, and Allergan; and serves on the editorial boards of Cephalalgia and the Journal of Headache and Pain. Aud Nome Dueland served on advisory boards for Lilly, Lundbeck, Novartis, and Teva; received lecture honoraria from Allergan-AbbVie, Lilly, Lundbeck, Novartis, Roche, and Teva; and received honoraria for consulting services from Allergan-AbbVie. Bianca Raffaelli has served on advisory boards for Novartis and Teva; has received lecture honoraria from Allergan, Lilly, Novartis, and Teva; has received research funding from Novartis; and is a member of the editorial boards of the Journal of Headache and Pain and Frontiers in Neurology.
Ethics Approval
The study was approved by the ethics committees of the three participating sites under local regulations (Berlin AE1/159/22, Pavia 0097925/21, and L’Aquila 34/2021).
Consent to Participate
Informed consent was obtained from all patients enrolled in Pavia and L’Aquila centers, while the document was not required in Berlin because of the retrospective nature of the study.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
LHO had full access to the data in this study and takes responsibility for the data and the accuracy of the data analysis. Study concept and design: RO, AND, BR, DM. Acquisition of data: LHO, RO, MMP, BR, DM. Analysis and interpretation of data: LHO, RO, MMP, BR, DM. Drafting of the manuscript: LHO, RO, MMP, UR, SS, CT BR, DM. Statistical analysis: LHO. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Overeem, L.H., Ornello, R., Pocora, M.M. et al. A Retrospective Real-Life Multicenter Study on Concurrent Oral Preventive Treatments in Patients with Chronic Migraine Treated with OnabotulinumtoxinA. CNS Drugs 37, 453–465 (2023). https://doi.org/10.1007/s40263-023-01001-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01001-y