Abstract
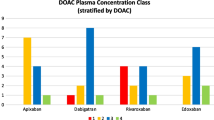
The use of direct oral anticoagulants (DOACs) is increasing because of their superior efficacy and safety compared with vitamin K antagonists. Pharmacokinetic drug interactions, particularly those involving cytochrome P450- mediated metabolism and P-glycoprotein transport, significantly affect the efficacy and safety of DOACs. In this article, we assess the effects of cytochrome P450- and P-glycoprotein-inducing antiseizure medications on DOAC pharmacokinetics in comparison to rifampicin. Rifampicin decreases to a varying extent the plasma exposure (area under the concentration–time curve) and peak concentration of each DOAC, consistent with its specific absorption and elimination pathways. For apixaban and rivaroxaban, rifampicin had a greater effect on the area under the concentration–time curve than on peak concentration. Therefore, using peak concentration to monitor DOAC concentrations may underestimate the effect of rifampicin on DOAC exposure. Antiseizure medications that are cytochrome P450 and P-glycoprotein inducers are commonly used with DOACs. Several studies have observed a correlation between the concomitant use of DOACs and enzyme-inducing antiseizure medications and DOAC treatment failure, for example, ischemic and thrombotic events. The European Society of Cardiology recommends avoiding this combination, as well as the combination of DOACs with levetiracetam and valproic acid, owing to a risk of low DOAC concentrations. However, levetiracetam and valproic acid are not cytochrome P450 or P-glycoprotein inducers, and the implications of their use with DOACs remain to be elucidated. Our comparative analysis suggests DOAC plasma concentration monitoring as a possible strategy to guide dosing owing to the predictable correlation between DOACs’ plasma concentration and effect. Patients taking concomitant enzyme-inducing antiseizure medications are at risk for low DOAC concentrations and subsequently, treatment failure and thus can benefit from DOAC concentration monitoring to prophylactically identify this risk.

Similar content being viewed by others
References
Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725. https://doi.org/10.1161/JAHA.116.003725.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel report. Chest. 2016;149:315–52.
Pundi KN, Perino AC, Fan J, Schmitt S, Kothari M, Szummer K, et al. Direct oral anticoagulant adherence of patients with atrial fibrillation transitioned from warfarin. J Am Heart Assoc. 2021;10: e020904.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62.
Derendorf H, Schmidt S, Rowland M, Tozer TN. Rowland and Tozer’s clinical pharmacokinetics and pharmacodynamics: concepts and applications. 5th ed. Philadelphia: Wolters Kluwer; 2020.
US Food and Drug Administration. Pradaxa prescribing information. 2010. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022512s000lbl.pdf. Accessed 20 Nov 2022.
US Food and Drug Administration. Xarelto prescribing information. 2011. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s000lbl.pdf. Accessed 20 Nov 2022.
US Food and Drug Administration. Eliquis prescribing information. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf. Accessed 20 Nov 2022.
US Food and Drug Administration. Savaysa prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf. Accessed 20 Nov 2022.
Center for Drug Evaluation and Research. FDA betrixaban clinical pharmacology and biopharmaceutics review. DRUGS@FDA data files. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208383Orig1s000ClinPharmR.pdf. Accessed 20 Nov 2022.
Kubitza D, Becka M, Roth A, Mueck W. Absence of clinically relevant interactions between rivaroxaban—an oral, direct Factor Xa inhibitor—and digoxin or atorvastatin in healthy subjects. J Int Med Res. 2012;40:1688–707.
Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S, et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs. 2013;13:331–42.
Škorňová I, Samoš M, Bolek T, Stančiaková L, Vádelová Ľ, Galajda P, et al. Does atorvastatin therapy change the anti-Xa activity in xabans-treated patients with atrial fibrillation? Pharmacol Res Perspect. 2021;9: e00730.
Soh XQ, Tan DS-Y, Chan ECY. Simvastatin, but not atorvastatin, is associated with higher peak rivaroxaban serum levels and bleeding: an Asian cohort study from Singapore. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07346-8.
Wu H-H, Chang S-H, Lee T-H, Tu H-T, Liu C-H, Chang T-Y. Concurrent use of statins decreases major bleeding and intracerebral hemorrhage in non-valvular atrial fibrillation patients taking direct oral anticoagulants: a nationwide cohort study. Front Cardiovasc Med. 2022;9: 969259.
Chang S-H, Chou I-J, Yeh Y-H, Chiou M-J, Wen M-S, Kuo C-T, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace. 2021;23:1612–76.
Chang K-H, Chen C-M, Wang C-L, Tu H-T, Huang Y-T, Wu H-C, et al. Major bleeding risk in patients with non-valvular atrial fibrillation concurrently taking direct oral anticoagulants and antidepressants. Front Aging Neurosci. 2022;14: 791285.
Bellia A, Della-Morte D, Di Daniele N, Lauro D. Drug interactions of direct oral anticoagulants in elderly patients with cardiometabolic diseases. Curr Res Pharmacol Drug Discov. 2021;2: 100029.
Chen C-M, Chang K-H, Wang C-L, Tu H-T, Huang Y-T, Wu H-C, et al. Major bleeding risk in atrial fibrillation patients co-medicated with non-vitamin K oral anticoagulants and antipsychotics. Front Pharmacol. 2022;13: 819878.
Härtter S, Koenen-Bergmann M, Sharma A, Nehmiz G, Lemke U, Timmer W, et al. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin: rifampicin decreases oral bioavailability of dabigatran etexilate. Br J Clin Pharmacol. 2012;74:490–500.
Center for Drug Evaluation and Research. FDA rivaroxaban clinical pharmacology biopharmaceutics review. DRUGS@FDA data files. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022406Orig1s000ClinPharmR.pdf. Accessed 19 Oct 2022.
Vakkalagadda B, Frost C, Byon W, Boyd RA, Wang J, Zhang D, et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of Factor Xa. Am J Cardiovasc Drugs. 2016;16:119–27.
Mendell J, Chen S, He L, Desai M, Parasramupria DA. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig. 2015;35:447–53.
Foerster KI, Hermann S, Mikus G, Haefeli WE. Drug–drug interactions with direct oral anticoagulants. Clin Pharmacokinet. 2020;59:967–80.
US Food and Drug Administration, FDA. Drug development and drug interactions. Table of substrates, inhibitors and inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers. Accessed 19 Oct 2022.
US Food and Drug Administration. Clinical drug interaction studies: cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 19 Oct 2022.
Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2:473–81.
Josephson CB, Wiebe S, Delgado-Garcia G, Gonzalez-Izquierdo A, Denaxas S, Sajobi TT, et al. Association of enzyme-inducing antiseizure drug use with long-term cardiovascular disease. JAMA Neurol. 2021;78:1367.
Chang C-S, Liao C-H, Lin C-C, Lane H-Y, Sung F-C, Kao C-H. Patients with epilepsy are at an increased risk of subsequent stroke: a population-based cohort study. Seizure. 2014;23:377–81.
Perlman A, Goldstein R, Choshen Cohen L, Hirsh-Raccah B, Hakimian D, Matok I, et al. Effect of enzyme-inducing antiseizure medications on the risk of sub-therapeutic concentrations of direct oral anticoagulants: a retrospective cohort study. CNS Drugs. 2021;35:305–16.
King PK, Stump TA, Walkama AM, Ash BM, Bowling SM. Management of phenobarbital and apixaban interaction in recurrent cardioembolic stroke. Ann Pharmacother. 2018;52:605–6.
Cole JL. Enzymatic deinduction phenomenon and clinical implications with a focus on direct-acting oral anticoagulants. Blood Coagul Fibrinolysis. 2020;31:283–6.
Di Gennaro L, Lancellotti S, De Cristofaro R, De Candia E. Carbamazepine interaction with direct oral anticoagulants: help from the laboratory for the personalized management of oral anticoagulant therapy. J Thromb Thrombolysis. 2019;48:528–31.
Stöllberger C, Finsterer J. Recurrent venous thrombosis under rivaroxaban and carbamazepine for symptomatic epilepsy. Neurol Neurochir Pol. 2017;51:194–6.
Burden T, Thompson C, Bonanos E, Medford AR. Lesson of the month 2: pulmonary embolism in a patient on rivaroxaban and concurrent carbamazepine. Clin Med. 2018;18:103–5.
Risselada AJ, Visser MJ, van Roon EN. Pulmonary embolism due to interaction between rivaroxaban and carbamazepine. Ned Tijdschr Geneeskd. 2013;157:A6568.
Hager N, Bolt J, Albers L, Wojcik W, Duffy P, Semchuk W. Development of left atrial thrombus after coadministration of dabigatran etexilate and phenytoin. Can J Cardiol. 2017;33:554.e13-554.e14.
Perlman A, Wanounou M, Goldstein R, Choshen Cohen L, Singer DE, Muszkat M. Ischemic and thrombotic events associated with concomitant Xa-inhibiting direct oral anticoagulants and antiepileptic drugs: analysis of the FDA Adverse Event Reporting System (FAERS). CNS Drugs. 2019;33:1223–8.
Gronich N, Stein N, Muszkat M. Association between use of pharmacokinetic-interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case-control study. Clin Pharmacol Ther. 2021;110:1526–36.
Werhahn KJ, Trinka E, Dobesberger J, Unterberger I, Baum P, Deckert-Schmitz M, et al. A randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsy. Epilepsia. 2015;56:450–9.
Ip BY, Ko H, Wong GL, Yip TC, Lau LH, Lau AY, et al. Thromboembolic risks with concurrent direct oral anticoagulants and antiseizure medications: a population-based analysis. CNS Drugs. 2022;36:1313–24.
Moerman L, wyffels L, Slaets D, Raedt R, Boon P, De Vos F. Antiepileptic drugs modulate P-glycoproteins in the brain: a mice study with 11C-desmethylloperamide. Epilepsy Res. 2011;94:18–25.
Mathy F-X, Dohin E, Bonfitto F, Pelgrims B. Drug-drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur Heart J. 2019;40:1571.
Levy RH, Ragueneau-Majlessi I, Baltes E. Repeated administration of the novel antiepileptic agent levetiracetam does not alter digoxin pharmacokinetics and pharmacodynamics in healthy volunteers. Epilepsy Res. 2001;46:93–9.
Paciullo F, Costa C, Gresele P. Rivaroxaban plasma levels and levetiracetam: a case report. Ann Intern Med. 2020;173:71–2.
Hole K, Wollmann BM, Nguyen C, Haslemo T, Molden E. Comparison of CYP3A4-inducing capacity of enzyme-inducing antiepileptic drugs Using 4β-hydroxycholesterol as biomarker. Ther Drug Monit. 2018;40:463–8.
Gjestad C, Huynh DK, Haslemo T, Molden E. 4β-Hydroxycholesterol correlates with dose but not steady-state concentration of carbamazepine: indication of intestinal CYP3A in biomarker formation? Br J Clin Pharmacol. 2016;81:269–76.
Bodin K, Bretillon L, Aden Y, Bertilsson L, Broomé U, Einarsson C, et al. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. J Biol Chem. 2001;276:38685–9.
Giner-Soriano M, Marsal JR, Gomez-Lumbreras A, Morros R. Risk of ischaemic stroke associated with antiepileptic drugs: a population-based case-control study in Catalonia. BMC Neurol. 2021;21:208.
Renoux C, Dell’Aniello S, Saarela O, Filion KB, Boivin J-F. Antiepileptic drugs and the risk of ischaemic stroke and myocardial infarction: a population-based cohort study. BMJ Open. 2015;5: e008365.
von Oertzen TJ, Trinka E, Bornstein NM. Levetiracetam and non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and epilepsy: a reasonable combination. Eur Heart J. 2019;40:3800–1.
Kurt S, Sumbul O, Aksoy D. Combination of non-vitamin K antagonist oral anticoagulants and antiepileptic drugs. Eur Heart J. 2019;40:1572.
Sarycheva T, Lavikainen P, Taipale H, Tiihonen J, Tanskanen A, Hartikainen S, et al. Antiepileptic drug use and mortality among community-dwelling persons with Alzheimer disease. Neurology. 2020;94:e2099–108.
Hughes RE, Tadi P, Bollu PC. TPA therapy. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK482376/. Accessed 24 Oct 2022.
Toorop MMA, Lijfering WM, Scheres LJJ. The relationship between DOAC levels and clinical outcomes: the measures tell the tale. J Thromb Haemost. 2020;18:3163–8.
Macha K, Marsch A, Siedler G, Breuer L, Strasser EF, Engelhorn T, et al. Cerebral ischemia in patients on direct oral anticoagulants: plasma levels are associated with stroke severity. Stroke. 2019;50:873–9.
Testa S, Paoletti O, Legnani C, Dellanoce C, Antonucci E, Cosmi B, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16:842–8.
Byon W, Sweeney K, Frost C, Boyd R. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism: subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol. 2017;6:340–9.
Leil TA, Frost C, Wang X, Pfister M, LaCreta F. Model-based exposure-response analysis of apixaban to quantify bleeding risk in special populations of subjects undergoing orthopedic surgery. CPT Pharmacometrics Syst Pharmacol. 2014;3: e136.
Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385:2288–95.
Johannessen Landmark C, Johannessen SI, Patsalos PN. Therapeutic drug monitoring of antiepileptic drugs: current status and future prospects. Expert Opin Drug Metab Toxicol. 2020;16:227–38.
Landmark CJ, Johannessen SI, Tomson T. Dosing strategies for antiepileptic drugs: from a standard dose for all to individualised treatment by implementation of therapeutic drug monitoring. Epileptic Disord. 2016;18:367–83.
Kahan BD, Keown P, Levy GA, Johnston A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther. 2002;24:330–50.
Tod MM, Padoin C, Petitjean O. Individualising aminoglycoside dosage regimens after therapeutic drug monitoring: simple or complex pharmacokinetic methods? Clin Pharmacokinet. 2001;40:803–14.
Winter ME. Basic clinical pharmacokinetics. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
Wang CL, Wu VC-C, Chang K-H, Tu H-T, Kuo C-F, Huang Y-T, et al. Assessing major bleeding risk in atrial fibrillation patients concurrently taking non-vitamin K antagonist oral anticoagulants and antiepileptic drugs. Eur Heart J Cardiovasc Pharmacother. 2020;6:147–54.
Van der Linden L, Hias J, Vanassche T. The value and limitations of new oral anticoagulant plasma level assessments. Eur Heart J Suppl. 2022;24:A32-41.
Drouet L, Bal dit Sollier C, Steiner T, Purrucker J. Measuring non-vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used? Int J Stroke. 2016;11:748–58.
Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Enzyme induction with AEDs Epilepsia. 2013;54:11–27.
Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–95.
European Medicines Agency. Eliquis: EPAR product Iiformation. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 20 Nov 2022.
Candeloro M, Eikelboom JW, Chan N, Bhagirath V, Douketis JD, Schulman S. Carbamazepine, phenytoin, and oral anticoagulants: drug-drug interaction and clinical events in a retrospective cohort. Res Pract Thromb Haemost. 2022;6: e12650.
Laureano M, Crowther M, Eikelboom J, Boonyawat K. Measurement of dabigatran drug levels to manage patients taking interacting drugs: a case report. Am J Med. 2016;129:e247–8.
Perlman A, Hochberg-Klein S, Choshen Cohen L, Dagan G, Hirsh-Raccah B, Horwitz E, et al. Management strategies of the interaction between direct oral anticoagulant and drug-metabolizing enzyme inducers. J Thromb Thrombolysis. 2019;47:590–5.
Sennesael A-L, Larock A-S, Hainaut P, Lessire S, Hardy M, Douxfils J, et al. The impact of strong inducers on direct oral anticoagulant levels. Am J Med. 2021;134:1295–9.
Giustozzi M, Mazzetti M, Paciaroni M, Agnelli G, Becattini C, Vedovati MC. Concomitant use of direct oral anticoagulants and antiepileptic drugs: a prospective cohort study in patients with atrial fibrillation. Clin Drug Investig. 2021;41:43–51.
Chin PKL, Wright DFB, Zhang M, Wallace MC, Roberts RL, Patterson DM, et al. Correlation between trough plasma dabigatran concentrations and estimates of glomerular filtration rate based on creatinine and cystatin C. Drugs R D. 2014;14:113–23.
Dagan G, Perlman A, Hochberg-Klein S, Kalish Y, Muszkat M. Managing direct oral anticoagulants in patients with antiepileptic medication. Can J Cardiol. 2018;34(1534):e1-3.
Wiggins BS, Northup A, Johnson D, Senfield J. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-7.
Sáez-Torres de Vicente M, Martínez Puig P, Valverde Toresano L. Ischemic stroke due to possible interaction of rivaroxaban with primidone in a patient with atrial fibrillation. Med Clin (Barc). 2021;156:255–6.
Robinson ZS, Arvin JP, Madding KL. Rivaroxaban failure in a patient taking oxcarbazepine. Ann Pharmacother. 2021;55:1302–3.
Serra W, Li Calzi M, Coruzzi P. Left atrial appendage thrombosis during therapy with rivaroxaban in elective cardioversion for permanent atrial fibrillation. Clin Pract. 2015;5. http://www.clinicsandpractice.org/index.php/cp/article/view/788. Accessed 1 Oct 2020.
Barbar S, Simonetto M, De Bon E, Scarano L, Caneve G, Simioni N. Direct oral anticoagulants and antiepileptic drugs: is there room for concurrent treatment? [abstract]. Res Pract Thromb Haemost. 2020. https://abstracts.isth.org/abstract/direct-oral-anticoagulants-and-antiepileptic-drugs-is-there-room-for-concurrent-treatment. Accessed 17 Jan 2023.
Stöllberger C, Finsterer J. Prolonged anticoagulant activity of rivaroxaban in a polymorbid elderly female with non-convulsive epileptic state. Heart Lung. 2014;43:262–3.
Langenbruch L, Meuth SG, Wiendl H, Mesters R, Möddel G. Clinically relevant interaction of rivaroxaban and valproic acid: a case report. Seizure. 2020;80:46–7.
Acknowledgments
This work is abstracted from the Ph.D. thesis of Rachel Goldstein in partial fulfillment of the Ph.D. degree requirements for The Hebrew University of Jerusalem. Meir Bialer (meirb@ekmd.huji.ac.il) and Mordechai Muszkat (muszkatm@hadassah.org.il) are co-corresponding authors for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by a research grant from the Estates Committee, Israel Ministry of Justice (proposal submitted to the Chief Scientist, Ministry of Health) to Mordechai Muszkat 2021–2024.
Conflicts of Interest
Meir Bialer received speaker’s or consultancy fees from Alkaloid, Boehringer Ingelheim, Clexio Bioscines, Guidepoint, Pharma Two B, Rekah-Vitamed, USWorldMeds, and Xenon Pharma. Rachel Goldstein, Aviya R. Jacobs, Lana Zighan, Naomi Gronich, and Mordechai Muszkat have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
RG, ARJ, LZ, NG, and MM produced an initial draft of the manuscript and conducted a literature search under guidance and supervision from MB and MM. RG, MB, and MB contributed to a critical evaluation of the data and to the revision and finalization of the manuscript. All authors have read and approved the final version of the manuscript, and agree to be accountable for the work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goldstein, R., Jacobs, A.R., Zighan, L. et al. Interactions Between Direct Oral Anticoagulants (DOACs) and Antiseizure Medications: Potential Implications on DOAC Treatment. CNS Drugs 37, 203–214 (2023). https://doi.org/10.1007/s40263-023-00990-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-00990-0