Abstract
Neurological diseases share common neuroinflammatory and oxidative stress pathways. Both phenotypic and molecular changes in microglia, astrocytes, and neurons contribute to the progression of disease and present potential targets for disease modification. Src family kinases (SFKs) are present in both neurons and glial cells and are upregulated following neurological insults in both human and animal models. In neurons, SFKs interact with post-synaptic protein domains to mediate hyperexcitability and neurotoxicity. SFKs are upstream of signaling cascades that lead to the modulation of neurotransmitter receptors and the transcription of pro-inflammatory cytokines as well as producers of free radicals through the activation of glia. Inducible nitric oxide synthase (iNOS/NOS-II) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2), the major mediators of reactive nitrogen/oxygen species (RNS/ROS) production in the brain, are also upregulated along with the pro-inflammatory cytokines following neurological insult and contribute to disease progression. Persistent neuronal hyperexcitability, RNS/ROS, and cytokines can exacerbate neurodegeneration, a common pathognomonic feature of the most prevalent neurological disorders such as Alzheimer's disease, Parkinson's disease, and epilepsy. Using a wide variety of preclinical disease models, inhibitors of the SFK-iNOS-NOX2 signaling axis have been tested to cure or modify disease progression. In this review, we discuss the SFK–iNOS–NOX2 signaling pathway and their inhibitors as potential CNS targets for major neurological diseases.
Plain Language Summary
Nerve cell death, oxidative stress, and inflammation of the brain are the most common pathological processes of many neurological diseases. These processes are mediated through changes in glial cells, the supporting cells in the brain, via several molecular pathways. Some of these pathways are potential drug targets for the mitigation of brain pathology. In this review, we focus on pathways involving Src family kinases, inducible nitric oxide synthase and nicotinamide adenine dinucleotide phosphate oxidase, and their inhibitors, which are promising agents for modifying neurological diseases.



Similar content being viewed by others
References
Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci. 2018;12:72. https://doi.org/10.3389/fncel.2018.00072.
Tang Y, Li X, Mao X. Editorial: linking neuroinflammation and glial phenotypic changes in neurological diseases. Front Cell Neurosci. 2019;13:542. https://doi.org/10.3389/fncel.2019.00542.
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG. Ramos-Escobar N. neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. 2019;10(SEP):1008. https://doi.org/10.3389/fphar.2019.01008.
Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–83. https://doi.org/10.1126/science.aag2590.
Gilhus NE, Deuschl G. Neuroinflammation—a common thread in neurological disorders. Nat Rev Neurol. 2019;15(8):429–30. https://doi.org/10.1038/s41582-019-0227-8.
Degan D, Ornello R, Tiseo C, Carolei A, Sacco S, Pistoia F. The role of inflammation in neurological disorders. Curr Pharm Des. 2018;24(14):1485–501. https://doi.org/10.2174/1381612824666180327170632.
Jäkel S, Dimou L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front Cell Neurosci. 2017. https://doi.org/10.3389/fncel.2017.00024.
Bachiller S, Jiménez-Ferrer I, Paulus A, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. https://doi.org/10.3389/fncel.2018.00488.
Von Bernhardi R, Eugenín-von Bernhardi L, Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 2015;7(JUN):124. https://doi.org/10.3389/fnagi.2015.00124.
Block ML. Neuroinflammation: modulating mighty microglia. Nat Chem Biol. 2014;10(12):988–9. https://doi.org/10.1038/nchembio.1691.
Kaufman AC, Salazar SV, Haas LT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol. 2015;77(6):953–71. https://doi.org/10.1002/ana.24394.
Sharma S, Carlson S, Puttachary S, et al. Role of the Fyn-PKCδ signaling in SE-induced neuroinflammation and epileptogenesis in experimental models of temporal lobe epilepsy. Neurobiol Dis. 2018;110:102–21. https://doi.org/10.1016/j.nbd.2017.11.008.
Nygaard HB. Targeting Fyn Kinase in Alzheimer’s Disease. Biol Psychiatry. 2018;83(4):369–76. https://doi.org/10.1016/j.biopsych.2017.06.004.
Sanz-Blasco S, Bordone MP, Damianich A, et al. The kinase Fyn as a novel intermediate in l-DOPA-induced dyskinesia in Parkinson’s Disease. Mol Neurobiol. 2018;55(6):5125–36. https://doi.org/10.1007/s12035-017-0748-3.
Liu D, Zhang X, Hu B, Ander BP. Src family kinases in brain edema after acute brain injury. In: Acta Neurochirurgica, Supplementum, vol 121. Vienna: Verlag; 2016. pp. 185–90. https://doi.org/10.1007/978-3-319-18497-5_33.
Liu DZ. Repurposing cancer drugs to treat neurological diseases—Src inhibitors as examples. Neural Regen Res. 2017;12(6):910–1. https://doi.org/10.4103/1673-5374.208569.
Van Dyck CH, Nygaard HB, Chen K, et al. Effect of AZD0530 on cerebral metabolic decline in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2019;76(10):1219–29. https://doi.org/10.1001/jamaneurol.2019.2050.
Purcell AL, Carew TJ. Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci. 2003;26(11):625–30. https://doi.org/10.1016/j.tins.2003.09.005.
Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48 REV. ISS. 7):7906–9. https://doi.org/10.1038/sj.onc.1208160.
Panicker N, Saminathan H, Jin H, et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of parkinson’s disease. J Neurosci. 2015;35(27):10058–77. https://doi.org/10.1523/JNEUROSCI.0302-15.2015.
Arias-Salvatierra D, Silbergeld EK, Acosta-Saavedra LC, Calderon-Aranda ES. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011;23(2):425–35. https://doi.org/10.1016/j.cellsig.2010.10.017.
Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–90. https://doi.org/10.1016/0891-0618(96)00148-2.
Boje KMK. Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763–76. https://doi.org/10.2741/1268.
Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–7. https://doi.org/10.1038/364535a0.
Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44(11):1873–86. https://doi.org/10.1016/j.freeradbiomed.2008.02.008.
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7(MAR):45. https://doi.org/10.3389/fncel.2013.00045.
Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15(2):313–26. https://doi.org/10.1016/0306-4522(85)90215-5.
Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–8. https://doi.org/10.1126/science.1190929.
Lenz KM, Nelson LH. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front Immunol. 2018;9(APR):698. https://doi.org/10.3389/fimmu.2018.00698.
Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8. https://doi.org/10.1126/science.1202529.
Sierra A, Paolicelli RC, Kettenmann H. Cien Años de Microglía: milestones in a century of microglial research. Trends Neurosci. 2019;42(11):778–92. https://doi.org/10.1016/j.tins.2019.09.004.
Ehlers MR, Todd RM. Genesis and maintenance of attentional biases: the role of the locus coeruleus-noradrenaline system. Neural Plast. 2017;1(1):2–3. https://doi.org/10.1155/2017.
Eyo UB, Wu LJ. Microglia: lifelong patrolling immune cells of the brain. Prog Neurobiol. 2019;179:101614. https://doi.org/10.1016/j.pneurobio.2019.04.003.
Engelhardt B. T cell migration into the central nervous system during health and disease: different molecular keys allow access to different central nervous system compartments. Clin Exp Neuroimmunol. 2010;1(2):79–93. https://doi.org/10.1111/j.1759-1961.2010.009.x.
da Fonseca ACC, Matias D, Garcia C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8(November):1–13. https://doi.org/10.3389/fncel.2014.00362.
Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis. 2017;107:41–56. https://doi.org/10.1016/j.nbd.2016.07.007.
Maiuolo J, Gliozzi M, Musolino V, et al. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19092693.
Mendes NF, Pansani AP, Carmanhães ERF, et al. The blood-brain barrier breakdown during acute phase of the Pilocarpine model of epilepsy is dynamic and time-dependent. Front Neurol. 2019;10:382. https://doi.org/10.3389/fneur.2019.00382.
Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53:1–6. https://doi.org/10.1111/j.1528-1167.2012.03696.x.
Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–84. https://doi.org/10.1523/JNEUROSCI.2614-05.2005.
Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–51. https://doi.org/10.1038/jcbfm.2011.197.
Cabezas R, Ávila M, Gonzalez J, et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci. 2014. https://doi.org/10.3389/fncel.2014.00211.
Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. https://doi.org/10.1038/nrn1824.
Liu CY, Yang Y, Ju WN, Wang X, Zhang HL. Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of Alzheimer’s disease. Front Cell Neurosci. 2018;12:193. https://doi.org/10.3389/fncel.2018.00193.
Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98(1):239–389. https://doi.org/10.1152/physrev.00042.2016.
Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci. 2015;35(41):13827–35. https://doi.org/10.1523/JNEUROSCI.2603-15.2015.
Prebil M, Jensen J, Zorec R, Kreft M. Astrocytes and energy metabolism. Arch Physiol Biochem. 2011;117(2):64–9. https://doi.org/10.3109/13813455.2010.539616.
Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–65. https://doi.org/10.1016/j.it.2008.05.002.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6):1–18. https://doi.org/10.1101/cshperspect.a020628.
Almad A, Maragakis NJ. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 2018;14(6):351–62. https://doi.org/10.1038/s41582-018-0010-2.
Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53(2):1181–94. https://doi.org/10.1007/s12035-014-9070-5.
Zhou T, Huang Z, Sun X, et al. Microglia polarization with M1/M2 phenotype changes in rd1 mouse model of retinal degeneration. Front Neuroanat. 2017;11:77. https://doi.org/10.3389/fnana.2017.00077.
Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–65. https://doi.org/10.1016/j.nurt.2010.05.014.
Liddelow SA, Barres BA. Review reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017. https://doi.org/10.1016/j.immuni.2017.06.006.
Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7. https://doi.org/10.1038/nature21029.
Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931–8. https://doi.org/10.1038/s41591-018-0051-5.
Putra M, Gage M, Sharma S, et al. Diapocynin, an NADPH oxidase inhibitor, counteracts diisopropylfluorophosphate-induced long-term neurotoxicity in the rat model. Ann N Y Acad Sci. 2020. https://doi.org/10.1111/nyas.14314.
Fernández-Arjona MM, Grondona JM, Granados-Durán P, Fernández-Llebrez P, López-Ávalos MD. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front Cell Neurosci. 2017;11:235. https://doi.org/10.3389/fncel.2017.00235.
Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. https://doi.org/10.1016/j.pneurobio.2015.05.003.
Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De GL. Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep. 2017;7(1):1–12. https://doi.org/10.1038/s41598-017-01747-8.
DePaula-Silva AB, Gorbea C, Doty DJ, et al. Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J Neuroinflammation. 2019;16(1):152. https://doi.org/10.1186/s12974-019-1545-x.
Puttachary S, Sharma S, Verma S, et al. 1400W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol Dis. 2016;93:184–200. https://doi.org/10.1016/j.nbd.2016.05.013.
Gage M, Golden M, Putra M, Sharma S, Thippeswamy T. Sex as a biological variable in the rat model of diisopropylfluorophosphate-induced long-term neurotoxicity. Ann N Y Acad Sci. 2020. https://doi.org/10.1111/nyas.14315.
Putra M, Sharma S, Gage M, et al. Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol Dis. 2020. https://doi.org/10.1016/j.nbd.2019.03.031.
Gómez-Nicola D, Fransen NL, Suzzi S, Hugh PV. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33(6):2481–93. https://doi.org/10.1523/JNEUROSCI.4440-12.2013.
Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275(3):305–15. https://doi.org/10.1016/j.expneurol.2015.03.020.
McLarnon JG. Microglial chemotactic signaling factors in Alzheimer’s disease. Am J Neurodegener Dis. 2012;1(3):199–204.
Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional microglia-neuron communication in health and disease. Front Cell Neurosci. 2018;12:323. https://doi.org/10.3389/fncel.2018.00323.
Lieu C, Kopetz S. The Src family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy. Clin Colorectal Cancer. 2010;9(2):89–94. https://doi.org/10.3816/CCC.2010.n.012.
Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23(48 REV. ISS. 7):7918–27. https://doi.org/10.1038/sj.onc.1208081.
Okada M. Regulation of the Src family kinases by Csk. Int J Biol Sci. 2012;8(10):1385–97. https://doi.org/10.7150/ijbs.5141.
Kojima N, Ishibashi H, Obata K, Kandel ER. Higher seizure susceptibility and enhanced tyrosine phosphorylation of N-methyl-d-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5(6):429-45. http://www.ncbi.nlm.nih.gov/pubmed/10489260. Accessed 15 Apr 2020.
Knox R, Jiang X. Fyn in neurodevelopment and ischemic brain injury. Dev Neurosci. 2015;37(4–5):311–20. https://doi.org/10.1159/000369995.
Lu YF, Kojima N, Tomizawa K, et al. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11(1):75–82. https://doi.org/10.1046/j.1460-9568.1999.00407.x.
Nygaard HB, Van Dyck CH, Strittmatter SM. Fyn kinase inhibition as a novel therapy for Alzheimer’s disease. Alzheimer’s Res Ther. 2014. https://doi.org/10.1186/alzrt238.
Salter MW, Kalia LV. SRC kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–28. https://doi.org/10.1038/nrn1368.
Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr Biol. 2003;13(1):9–17. https://doi.org/10.1016/S0960-9822(02)01397-0.
Jin DZ, Mao LM, Wang JQ. An essential role of fyn in the modulation of metabotropic glutamate receptor 1 in neurons. eNeuro. 2017. https://doi.org/10.1523/ENEURO.0096-17.2017.
Lu X, Hu X, Song L, et al. The SH2 domain is crucial for function of Fyn in neuronal migration and cortical lamination. BMB Rep. 2015;48(2):97–102. https://doi.org/10.5483/BMBRep.2015.48.2.067.
Suh YH, Chang K, Roche KW. Metabotropic glutamate receptor trafficking. Mol Cell Neurosci. 2018;91:10–24. https://doi.org/10.1016/j.mcn.2018.03.014.
Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279(1):12–9. https://doi.org/10.1111/j.1742-4658.2011.08391.x.
Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci USA. 1999;96(2):435–40. https://doi.org/10.1073/pnas.96.2.435.
Grant SGN, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258(5090):1903–10. https://doi.org/10.1126/science.1361685.
Lee G, Thangavel R, Sharma VM, et al. Phosphorylation of Tau by Fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24(9):2304–12. https://doi.org/10.1523/JNEUROSCI.4162-03.2004.
Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–97. https://doi.org/10.1016/j.cell.2010.06.036.
Putra M, Puttachary S, Liu G, Lee G, Thippeswamy T. Fyn-tau ablation modifies PTZ-induced seizures and post-seizure hallmarks of early epileptogenesis. Front Cell Neurosci. 2020;14:592374. https://doi.org/10.3389/fncel.2020.592374.
Lewerenz J, Maher P. Chronic glutamate toxicity in neurodegenerative diseases-What is the evidence? Front Neurosci. 2015;9(22):469. https://doi.org/10.3389/fnins.2015.00469.
Cain DP, Grant SGN, Saucier D, Hargreaves EL, Kandel ER. Fyn tyrosine kinase is required for normal amygdala kindling. Epilepsy Res. 1995;22(2):107–14. https://doi.org/10.1016/0920-1211(95)00029-1.
Moussa RC, Ikeda-Douglas CJ, Thakur V, Milgram NW, Gurd JW. Seizure activity results in increased tyrosine phosphorylation of the N-methyl-d-aspartate receptor in the hippocampus. Mol Brain Res. 2001;95(1–2):36–47. https://doi.org/10.1016/S0169-328X(01)00231-5.
Nakazawa T, Komai S, Tezuka T, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J Biol Chem. 2001;276(1):693–9. https://doi.org/10.1074/jbc.M008085200.
Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–53. https://doi.org/10.1073/pnas.95.11.6448.
Boehm SL, Peden L, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters sensitivity to GABAergic drugs: dependence on β2/β3 GABAA receptor subunits. J Pharmacol Exp Ther. 2004;309(3):1154–9. https://doi.org/10.1124/jpet.103.064444.
Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABAA receptor γ2 subunit. Mol Cell Neurosci. 2010;44(2):129–34. https://doi.org/10.1016/j.mcn.2010.03.002.
Hsieh HL, Wang HH, Wu CY, Tung WH, Yang CM. Lipoteichoic acid induces matrix metalloproteinase-9 expression via transactivation of PDGF receptors and NF-kappaB activation in rat brain astrocytes. Neurotox Res. 2010;17(4):344–59. https://doi.org/10.1007/s12640-009-9111-4.
Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009. https://doi.org/10.1101/cshperspect.a001271.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23.
Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015. https://doi.org/10.3389/fnmol.2015.00077.
Anrather J, Racchumi G, Iadecola C. NF-κB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657–67. https://doi.org/10.1074/jbc.M506172200.
Socodato R-S, Portugal CC, Domith I, et al. rc function is necessary and sufficient for triggering microglial cell activation. Glia. 2015;63(3):497–511. https://doi.org/10.1002/glia.22767.
Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer’s disease. J Neuroinflammation. 2012;9(1):563. https://doi.org/10.1186/1742-2094-9-117.
Li Z, Li W, Li Q, Tang M. Extracellular nucleotides and adenosine regulate microglial motility and their role in cerebral ischemia. Acta Pharm Sin B. 2013;3(4):205–12. https://doi.org/10.1016/j.apsb.2013.06.003.
Fan Y, Xie L, Chung CY. Signaling pathways controlling microglia chemotaxis. Mol Cells. 2017;40(3):163–8. https://doi.org/10.14348/molcells.2017.0011.
Lee SH, Hollingsworth R, Kwon HY, Lee N, Chung CY. β-arrestin 2-dependent activation of ERK1/2 is required for ADP-induced paxillin phosphorylation at Ser83 and microglia chemotaxis. Glia. 2012;60(9):1366–77. https://doi.org/10.1002/glia.22355.
Ahluwalia MS, de Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298(2):139–49. https://doi.org/10.1016/j.canlet.2010.08.014.
Wang JYJ. The capable ABL: what is its biological function? Mol Cell Biol. 2014;34(7):1188–97. https://doi.org/10.1128/MCB.01454-13.
Chong YP, Chan AS, Chan KC, et al. C-terminal Src kinase-homologous kinase (CHK), a unique inhibitor inactivating multiple active conformations of Src family tyrosine kinases. J Biol Chem. 2006;281(44):32988–99. https://doi.org/10.1074/jbc.M602951200.
Chong YP, Mulhern TD, Cheng HC. C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)—endogenous negative regulators of Src-family protein kinases. Growth Factors. 2005;23(3):233–44. https://doi.org/10.1080/08977190500178877.
Ölgen S, Isgör YG, Çoban T. Synthesis and activity of novel 5-substituted pyrrolo[2,3-d]pyrimidine analogues as pp60c-Src tyrosine kinase inhibitors. Arch Pharm (Weinheim). 2008;341(2):113–20. https://doi.org/10.1002/ardp.200700141.
Breen ME, Steffey ME, Lachacz EJ, Kwarcinski FE, Fox CC, Soellner MB. Substrate activity screening with kinases: discovery of small-molecule substrate-competitive c-Src inhibitors. Angew Chem Int Ed. 2014;53(27):7010–3. https://doi.org/10.1002/anie.201311096.
Shim HJ, Kim HI, Lee ST. The associated pyrazolopyrimidines PP1 and PP2 inhibit protein tyrosine kinase 6 activity and suppress breast cancer cell proliferation. Oncol Lett. 2017;13(3):1463–9. https://doi.org/10.3892/ol.2017.5564.
Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36(6):492–500. https://doi.org/10.1016/j.ctrv.2010.02.015.
Kantarjian H, O’Brien S, Cortes J, et al. Analysis of the impact of imatinib mesylate therapy on the prognosis of patients with Philadelphia chromosome-positive chronic myelogenous leukemia treated with interferon-alpha regimens for early chronic phase. Cancer. 2003;98(7):1430–7. https://doi.org/10.1002/cncr.11665.
Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2015;201:27–65. https://doi.org/10.1007/978-3-642-54490-3_2.
Schiff, D. Jann S. Dasatinib in recurrent glioblastoma: failure as a teacher. Neuro Oncol. 2015;17(7):910–11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5762007/. Accessed 27 May 2020.
Shah NP, Wallis N, Farber HW, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol. 2015;90(11):1060–4. https://doi.org/10.1002/ajh.24174.
Waller CF. Imatinib Mesylate. Recent Results Cancer Res. 2010;184:3–20. https://doi.org/10.1007/978-3-642-01222-8_1.
Mittapalli RK, Chung AH, Parrish KE, et al. ABCG2 and ABCB1 limit the efficacy of dasatinib in a PDGF-B-driven brainstem glioma model. Mol Cancer Ther. 2016;15(5):819–29. https://doi.org/10.1158/1535-7163.MCT-15-0093.
Kast RE, Focosi D. Three paths to better tyrosine kinase inhibition behind the blood-brain barrier in treating chronic myelogenous leukemia and glioblastoma with imatinib. Transl Oncol. 2010;3(1):13–5. https://doi.org/10.1593/tlo.09280.
Tyryshkin A, Bhattacharya A, Eissa NT. Src kinase is a novel therapeutic target in lymphangioleiomyomatosis. Cancer Res. 2014;74(7):1996–2005. https://doi.org/10.1158/0008-5472.CAN-13-1256.
Minami SS, Clifford TG, Hoe HS, Matsuoka Y, Rebeck GW. Fyn knock-down increases Aβ, decreases phospho-tau, and worsens spatial learning in 3×Tg-AD mice. Neurobiol Aging. 2012;33(4):825.e15-825.e24. https://doi.org/10.1016/j.neurobiolaging.2011.05.014.
Stein PL, Vogel H, Soriano P. Combined deficiencies of src, fyn, and yes tyrosine kinases in mutant mice. Genes Dev. 1994;8(17):1999–2007. https://doi.org/10.1101/gad.8.17.1999.
Huerta PT, Scearce KA, Farris SM, Empson RM, Prusky GT. Preservation of spatial learning in fyn tyrosine kinase knockout mice. NeuroReport. 1996;7(10):1685–9. https://doi.org/10.1097/00001756-199607080-00032.
Yang H, Wang L, Zang C, et al. Src inhibition attenuates neuroinflammation and protects dopaminergic neurons in Parkinson’s disease models. Front Neurosci. 2020;14:45. https://doi.org/10.3389/fnins.2020.00045.
Galanis E, Anderson SK, Twohy EL, et al. A phase 1 and randomized, placebo-controlled phase 2 trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma: Alliance/North Central Cancer Treatment Group N0872. Cancer. 2019;125(21):3790–800. https://doi.org/10.1002/cncr.32340.
Cortes JE, Jiang Q, Wang J, et al. Dasatinib vs. imatinib in patients with chronic myeloid leukemia in chronic phase (CML-CP) who have not achieved an optimal response to 3 months of imatinib therapy: the DASCERN randomized study. Leukemia. 2020. https://doi.org/10.1038/s41375-020-0805-1.
Huang X, Jiang Q, Hu J, et al. Long-term safety of dasatinib in chinese chronic phase chronic myeloid leukemia patients with imatinib-resistance or -intolerance: results from a 6-year follow-up of a multicenter phase II study. Blood. 2016;128(22):1928–1928. https://doi.org/10.1182/blood.v128.22.1928.1928.
Lassman AB, Pugh SL, Gilbert MR, Aldape KD, Geinoz S, Buemer JH, Christner SM, Ritsuko R, DeAngelis LM, Gaur R, Yossef E, Wagner H, Won M, Mehta M. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol. 2015;17(7):992–8.
Chamoun K, Rabinovich E, Baer L, Fastenau P, de Lima M. A case of neurocognitive deficit strongly related to dasatinib therapy. Hematol Transfus Cell Ther. 2020;42(1):80–2. https://doi.org/10.1016/j.htct.2019.01.003.
Baselga J, Cervantes A, Martinelli E, et al. Phase I safety, pharmacokinetics, and inhibition of src activity study of saracatinib in patients with solid tumors. Clin Cancer Res. 2010;16(19):4876–83. https://doi.org/10.1158/1078-0432.CCR-10-0748.
Fujisaka Y, Onozawa Y, Kurata T, et al. First report of the safety, tolerability, and pharmacokinetics of the Src kinase inhibitor saracatinib (AZD0530) in Japanese patients with advanced solid tumours. Invest New Drugs. 2013;31(1):108–14. https://doi.org/10.1007/s10637-012-9809-7.
Nygaard HB, Wagner AF, Bowen GS, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimer’s Res Ther. 2015. https://doi.org/10.1186/s13195-015-0119-0.
Mattson MP, Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107(3):247–54. https://doi.org/10.1172/JCI11916.
Hatano E, Bennett BL, Manning AM, Qian T, Lemasters JJ, Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-a. Gastroenterology. 2001;120(5):1251–62.
Srinivasan M, Lahiri DK. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer’s disease and multiple sclerosis. Expert Opin Ther Targets. 2015;19(4):471–87. https://doi.org/10.1517/14728222.2014.989834.
Mattson MP, Meffert MK. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–60. https://doi.org/10.1038/sj.cdd.4401837.
Sonar SA, Lal G. The iNOS activity during an immune response controls the CNS pathology in experimental autoimmune encephalomyelitis. Front Immunol. 2019;10(APR):710. https://doi.org/10.3389/fimmu.2019.00710.
Matrone C, Pignataro G, Molinaro P, et al. HIF-1α reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem. 2004;90(2):368–78. https://doi.org/10.1111/j.1471-4159.2004.02483.x.
Beest V. Statin users risk heart attacks by dropping treatment or taking l ow doses Doctors must emphasise importance of compl ying with treatment say researchers. Heart. 2006;91(December):250–6. https://doi.org/10.1093/eurheartj.
Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418(3):291–6. https://doi.org/10.1016/s0014-5793(97)01397-5.
Xian M, Fujiwara N, Wen Z, et al. Novel substrates for nitric oxide synthases. Bioorg Med Chem. 2002;10(9):3049–55. https://doi.org/10.1016/s0968-0896(02)00155-4.
Forstermann U, Sessa W. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37. https://doi.org/10.1093/eurheartj/ehr304.
Walker G, Pfeilschifter J, Kunz D. Mechanisms of suppression of inducible nitric-oxide synthase (iNOS) expression in interferon (IFN)-γ-stimulated RAW 264.7 cells by dexamethasone. Evidence for glucocorticoid-induced degradation of iNOS protein by calpain as a key step in post-transcriptional regulation. J Biol Chem. 1997;272(26):16679–87. https://doi.org/10.1074/jbc.272.26.16679.
Knott AB, Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxidants Redox Signal. 2009;11(3):541–53. https://doi.org/10.1089/ars.2008.2234.
Sharma S, Puttachary S, Thippeswamy T. Glial source of nitric oxide in epileptogenesis: a target for disease modification in epilepsy. J Neurosci Res. 2017. https://doi.org/10.1002/jnr.24205.
Fuentes E, Gibbins JM, Holbrook LM, Palomo I. NADPH oxidase 2 (NOX2): a key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc Med. 2018;28(7):429–34. https://doi.org/10.1016/j.tcm.2018.03.001.
Sharma S, Puttachary S, Thippeswamy T. Glial source of nitric oxide in epileptogenesis: a target for disease modification in epilepsy. J Neurosci Res. 2019;97(11):1363–77. https://doi.org/10.1002/jnr.24205.
Raad H, Paclet MH, Boussetta T, et al. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23(4):1011–22. https://doi.org/10.1096/fj.08-114553.
Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12(1):5–23. https://doi.org/10.1038/cmi.2014.89.
Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 2013;10(1):155. https://doi.org/10.1186/1742-2094-10-155.
Zhang L, Wu J, Duan X, et al. NADPH oxidase: a potential target for treatment of stroke. Oxid Med Cell Longev. 2016. https://doi.org/10.1155/2016/5026984.
Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51(1):171–8. https://doi.org/10.1016/j.freeradbiomed.2011.03.025.
Tannich F, Tlili A, Pintard C, et al. Activation of the phagocyte NADPH oxidase/NOX2 and myeloperoxidase in the mouse brain during pilocarpine-induced temporal lobe epilepsy and inhibition by ketamine. Inflammopharmacology. 2020;28(2):487–97. https://doi.org/10.1007/s10787-019-00655-9.
Belarbi K, Cuvelier E, Destée A, Gressier B, Chartier-Harlin MC. NADPH oxidases in Parkinson’s disease: a systematic review. Mol Neurodegener. 2017;12(1):1–18. https://doi.org/10.1186/s13024-017-0225-5.
Ma MW, Wang J, Dhandapani KM, Wang R, Brann DW. NADPH oxidases in traumatic brain injury—promising therapeutic targets? Redox Biol. 2018;16:285–93. https://doi.org/10.1016/j.redox.2018.03.005.
Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2011;15(7):1957–97. https://doi.org/10.1089/ars.2010.3586.
Zitka O, Skalickova S, Gumulec J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4(6):1247–53. https://doi.org/10.3892/ol.2012.931.
Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci. 2013;14(10):21021–44. https://doi.org/10.3390/ijms141021021.
Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904–11. https://doi.org/10.1046/j.1432-1327.2000.01595.x.
Johnson WM, Wilson-Delfosse AL, Mieyal JJ. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012;4(10):1399–440. https://doi.org/10.3390/nu4101399.
Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxidants Redox Signal. 2018;29(17):1727–45. https://doi.org/10.1089/ars.2017.7342.
Mcwalter GK, Higgins LG, Mclellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H: quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by Broccoli Seeds and Isothiocyanates. J Nutr. 2004;134(12):3499S-3506S. https://doi.org/10.1093/jn/134.12.3499S.
Branca C, Ferreira E, Nguyen T-V, Doyle K, Caccamo A, Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2017;26(24):4823–35. https://doi.org/10.1093/hmg/ddx361.
Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53(1):401–26. https://doi.org/10.1146/annurev-pharmtox-011112-140320.
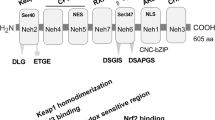
Kaspar JW, Jaiswal AK. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011;25(3):1076–87. https://doi.org/10.1096/fj.10-171553.
Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90. https://doi.org/10.1016/j.cellsig.2012.01.008.
Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–9. https://doi.org/10.1016/j.tplants.2016.08.002.
Lind M, Hayes A, Caprnda M, et al. Inducible nitric oxide synthase: good or bad? Biomed Pharmacother. 2017;93:370–5. https://doi.org/10.1016/j.biopha.2017.06.036.
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS. The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp Neurol. 2015;263:235–43. https://doi.org/10.1016/j.expneurol.2014.10.017.
Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424(1–2):37–49. https://doi.org/10.1016/s0027-5107(99)00006-8.
Pérez-Asensio FJ, Hurtado O, Burguete MC, et al. Inhibition of iNOS activity by 1400W decreases glutamate release and ameliorates stroke outcome after experimental ischemia. Neurobiol Dis. 2005;18(2):375–84. https://doi.org/10.1016/j.nbd.2004.10.018.
Ahn J-Y. Neuroprotection signaling of nuclear Akt in neuronal cells. Exp Neurobiol. 2014;23(3):200–6. https://doi.org/10.5607/en.2014.23.3.200.
Kwak YD, Ma T, Diao S, et al. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener. 2010;5(1):49. https://doi.org/10.1186/1750-1326-5-49.
Qu J, Nakamura T, Holland EA, McKercher SR, Lipton SA. S-nitrosylation of Cdk5: potential implications in amyloid-β-related neurotoxicity in Alzheimer disease. Prion. 2012;6(4):364–70. https://doi.org/10.4161/pri.21250.
Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto SI, Lipton SA. Aberrant Protein S-nitrosylation in neurodegenerative diseases. Neuron. 2013;78(4):596–614. https://doi.org/10.1016/j.neuron.2013.05.005.
Mukherjee P, Cinelli MA, Kang S, Silverman RB. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2014;43(19):6814–38. https://doi.org/10.1039/c3cs60467e.
Janakiram NB, Rao CV. INOS-selective inhibitors for cancer prevention: promise and progress. Future Med Chem. 2012;4(17):2193–204. https://doi.org/10.4155/fmc.12.168.
Víteček J, Lojek A, Valacchi G, Kubala L. Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediators Inflamm. 2012;2012:318087. https://doi.org/10.1155/2012/318087.
Kopincová J, Púzserová A, Bernátová I. l-NAME in the cardiovascular system—nitric oxide synthase activator? Pharmacol Rep. 2012;64(3):511–20. https://doi.org/10.1016/S1734-1140(12)70846-0.
Peterson DA, Peterson DC, Archer S, Weir EK. The non specificity of specific nitric oxide synthase inhibitors. Biochem Biophys Res Commun. 1992;187(2):797–801. https://doi.org/10.1016/0006-291X(92)91266-S.
Liu T, Zhang M, Mukosera GT, et al. l-NAME releases nitric oxide and potentiates subsequent nitroglycerin-mediated vasodilation. Redox Biol. 2019. https://doi.org/10.1016/j.redox.2019.101238.
Corbett JA, McDaniel ML. The use of aminoguanidine, a selective iNOS inhibitor, to evaluate the role of nitric oxide in the development of autoimmune diabetes. Methods A Companion Methods Enzymol. 1996;10(1):21–30. https://doi.org/10.1006/meth.1996.0074.
Hou FF, Boyce J, Chertow GM, Kay J, Owen WF. Aminoguanidine inhibits advanced glycation end products formation on β2-microglobulin. J Am Soc Nephrol. 1998;9(2):277–83.
Garvey EP, Oplinger JA, Furfine ES, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272(8):4959–63. https://doi.org/10.1074/JBC.272.8.4959.
Dover AR, Chia S, Ferguson JW, et al. Inducible nitric oxide synthase activity does not contribute to the maintenance of peripheral vascular tone in patients with heart failure. Clin Sci. 2006;111(4):275–80. https://doi.org/10.1042/CS20060104.
Ferguson JW, Dover AR, Chia S, Cruden NLM, Hayes PC, Newby DE. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut. 2006;55(4):542–6. https://doi.org/10.1136/gut.2005.076562.
Zhu Y, Nikolic D, Van Breemen RB, Silverman RB. Mechanism of inactivation of inducible nitric oxide synthase by amidines. Irreversible enzyme inactivation without inactivator modification. J Am Chem Soc. 2005;127(3):858–68. https://doi.org/10.1021/ja0445645.
Bretscher LE, Li H, Poulos TL, Griffith OW. Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(Iminoalkyl)- and N 5-(Iminoalkenyl)-ornithines. J Biol Chem. 2003;278(47):46789–97. https://doi.org/10.1074/jbc.M306787200.
Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci USA. 1998;95(18):10966–71. https://doi.org/10.1073/pnas.95.18.10966.
Sharma B, Singh N. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, convalesce behavior and biochemistry of hypertension induced vascular dementia in rats. Pharmacol Biochem Behav. 2013;103(4):821–30. https://doi.org/10.1016/j.pbb.2012.11.011.
Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50(5):633–40. https://doi.org/10.1016/j.freeradbiomed.2010.12.026.
Cosgrave AS, McKay JS, Bubb V, Morris R, Quinn JP, Thippeswamy T. Regulation of activity-dependent neuroprotective protein (ADNP) by the NO-cGMP pathway in the hippocampus during kainic acid-induced seizure. Neurobiol Dis. 2008;30(3):281–92. https://doi.org/10.1016/j.nbd.2008.02.005.
Beamer E, Otahal J, Sills GJ, Thippeswamy T. N w-Propyl-l-arginine (L-NPA) reduces status epilepticus and early epileptogenic events in a mouse model of epilepsy: behavioural, EEG and immunohistochemical analyses. Eur J Neurosci. 2012;36(9):3194–203. https://doi.org/10.1111/j.1460-9568.2012.08234.x.
Rehni AK, Singh TG, Kalra R, Singh N. Pharmacological inhibition of inducible nitric oxide synthase attenuates the development of seizures in mice. Nitric Oxide Biol Chem. 2009;21(2):120–5. https://doi.org/10.1016/j.niox.2009.06.001.
Tse K, Hammond D, Simpson D, et al. The impact of postsynaptic density 95 blocking peptide (Tat-NR2B9c) and an iNOS inhibitor (1400W) on proteomic profile of the hippocampus in C57BL/6J mouse model of kainate-induced epileptogenesis. J Neurosci Res. 2019;97(11):1378–92. https://doi.org/10.1002/jnr.24441.
Jarvinen K, Vuolteenaho K, Nieminen R, Moilanen T, Knowles RG, Moilanen E. Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody arra. Clin Exp Rheumatol. 2008;26(2):275–82.
Staunton CA, Barrett-Jolley R, Djouhri L, Thippeswamy T. Inducible nitric oxide synthase inhibition by 1400W limits pain hypersensitivity in a neuropathic pain rat model. Exp Physiol. 2018;103(4):535–44. https://doi.org/10.1113/EP086764.
Putra M, Sharma S, Gage M, et al. Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol Dis. 2019. https://doi.org/10.1016/J.NBD.2019.03.031.
Barua A, Standen NB, Galĩanes M. Dual role of nNOS in ischemic injury and preconditioning. BMC Physiol. 2010;10(1):15. https://doi.org/10.1186/1472-6793-10-15.
Talukder MAH, Yang F, Shimokawa H, Zweier JL. eNOS is required for acute in vivo ischemic preconditioning of the heart: effects of ischemic duration and sex. Am J Physiol Circ Physiol. 2010;299(2):H437–45. https://doi.org/10.1152/ajpheart.00384.2010.
Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide Biol Chem. 2011;25:70–80. https://doi.org/10.1016/j.niox.2011.01.003.
Tegtmeier F, Schinzel R, Beer R, et al. Efficacy of ronopterin (VAS203) in patients with moderate and severe traumatic brain injury (NOSTRA phase III trial): study protocol of a confirmatory, placebo-controlled, randomised, double blind, multi-centre study. Trials. 2020;21(1):80. https://doi.org/10.1186/s13063-019-3965-4.
Stover JF, Belli A, Boret H, et al. Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized phase iia trial (NOSTRA). J Neurotrauma. 2014;31(19):1599–606. https://doi.org/10.1089/neu.2014.3344.
Chen Y, Brennan-Minnella AM, Sheth S, El-Benna J, Swanson RA. Tat-NR2B9c prevents excitotoxic neuronal superoxide production. J Cereb Blood Flow Metab. 2015;35(5):739–42. https://doi.org/10.1038/jcbfm.2015.16.
Hill MD, Martin RH, Mikulis D, et al. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11(11):942–50. https://doi.org/10.1016/S1474-4422(12)70225-9.
Ballarin B, Tymianski M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol Sin. 2018;39(5):661–8. https://doi.org/10.1038/aps.2018.5.
Prado CM, Martins MA, Tibério IFLC. Nitric Oxide in Asthma Physiopathology. ISRN Allergy. 2011. https://doi.org/10.5402/2011/832560.
Brindicci C, Ito K, Torre O, Barnes PJ, Kharitonov SA. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest. 2009;135(2):353–67. https://doi.org/10.1378/chest.08-0964.
Hansel TT, Kharitonov SA, Donnelly LE, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17(10):1298–300. https://doi.org/10.1096/fj.02-0633fje.
Singh D, Richards D, Knowles RG, et al. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176(10):988–93. https://doi.org/10.1164/rccm.200704-588OC.
Barua S, Kim JY, Yenari MA, Lee JE. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep. 2019;7:59–69. https://doi.org/10.1016/j.ibror.2019.07.1721.
Diebold BA, Smith SME, Li Y, Lambeth JD. NOX2 as a target for drug development: Indications, possible complications, and progress. Antioxidants Redox Signal. 2015;23(5):375–405. https://doi.org/10.1089/ars.2014.5862.
Beukelman CJ, van den Worm E, Henriette C, van Ufford Q, Kroes BH, van den Berg AJJ. Discovery of new anti-inflammatory drugs from plant origins. Ann Gastroenterol. 2002;15(4):320–3.
Wang Q, Smith RE, Luchtefeld R, et al. Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 2008;15(6–7):496–503. https://doi.org/10.1016/j.phymed.2007.09.019.
Marín M, Gimeno C, Giner RM, Ríos JL, Máñez S, Recio MC. Influence of dimerization of apocynin on its effects in experimental colitis. J Agric Food Chem. 2017;65(20):4083–91. https://doi.org/10.1021/acs.jafc.7b00872.
Macías-Pérez ME, Martínez-Ramos F, Padilla-Martínez II, et al. Ethers and esters derived from apocynin avoid the interaction between p47phox and p22phox subunits of NADPH oxidase: evaluation in vitro and in silico. Biosci Rep. 2013;33(4):605–16. https://doi.org/10.1042/BSR20130029.
Ghosh A, Kanthasamy A, Joseph J, et al. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J Neuroinflammation. 2012;9(1):711. https://doi.org/10.1186/1742-2094-9-241.
Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta Mol Basis Dis. 2014;1842(8):1282–94. https://doi.org/10.1016/j.bbadis.2013.09.007.
Ghosh A, Langley MR, Harischandra DS, et al. Mitoapocynin treatment protects against neuroinflammation and dopaminergic neurodegeneration in a preclinical animal model of Parkinson’s Disease. J Neuroimmune Pharmacol. 2016;11(2):259–78. https://doi.org/10.1007/s11481-016-9650-4.
Langley M, Ghosh A, Charli A, et al. Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in mitopark transgenic mice. Antioxidants Redox Signal. 2017;27(14):1048–66. https://doi.org/10.1089/ars.2016.6905.
Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Diphenyleneiodonium acutely inhibits reactive oxygen species production by mitochondrial complex I during reverse, but not forward electron transport. Biochim Biophys Acta Bioenerg. 2008;1777(5):397–403. https://doi.org/10.1016/j.bbabio.2008.03.005.
Augsburger F, Filippova A, Rasti D, et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019. https://doi.org/10.1016/j.redox.2019.101272.
Huang WY, Lin S, Chen HY, et al. NADPH oxidases as potential pharmacological targets against increased seizure susceptibility after systemic inflammation. J Neuroinflammation. 2018. https://doi.org/10.1186/s12974-018-1186-5.
Shekh-Ahmad T, Lieb A, Kovac S, et al. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019;26:101278. https://doi.org/10.1016/j.redox.2019.101278.
Stefanska J, Sarniak A, Wlodarczyk A, et al. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res. 2012;38(2):90–9. https://doi.org/10.3109/01902148.2011.649823.
Peters EA, Hiltermann JTN, Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med. 2001;31(11):1442–7. https://doi.org/10.1016/S0891-5849(01)00725-0.
DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Ren Physiol. 2014;306(12):F1499. https://doi.org/10.1152/ajprenal.00058.2014.
Dalekos G, Invernizzi P, Nevens F, et al. GS-02-Efficacy of GKT831 in patients with primary biliary cholangitis and inadequate response to ursodeoxycholic acid: Interim efficacy results of a phase 2 clinical trial. J Hepatol. 2019;70(1):e1–2. https://doi.org/10.1016/s0618-8278(19)30002-7.
Kim JY, Park J, Lee JE, Yenari MA. NOX inhibitors—a promising avenue for ischemic stroke. Exp Neurobiol. 2017;26(4):195–205. https://doi.org/10.5607/en.2017.26.4.195.
Cotgreave IA, Duddy SK, Kass GEN, Thompson D, Moldéus P. Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of nadph oxidase and protein kinase C? Biochem Pharmacol. 1989;38(4):649–56. https://doi.org/10.1016/0006-2952(89)90211-6.
Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29(1):12–7. https://doi.org/10.1161/01.STR.29.1.12.
Azad GK, Tomar RS. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol Biol Rep. 2014;41(8):4865–79. https://doi.org/10.1007/s11033-014-3417-x.
Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. https://doi.org/10.1016/S1474-4422(18)30499-X.
Dagdeviren M. Role of nitric oxide synthase in normal brain function and pathophysiology of neural diseases. In: Nitric oxide synthase simple enzyme complex roles. InTech; 2017. https://doi.org/10.5772/67267.
Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus—Is this good or bad? Histol Histopathol. 2006;21(4–6):445–58. https://doi.org/10.14670/HH-21.445.
Acknowledgments
We would like to acknowledge Mica Post (Medical Illustrators, Department of Biomedical Sciences, Iowa State University, Ames, Iowa, USA) who drew Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Support to TT is from the National Institute of Health, NINDS/CounterACT program (NS099007, NS110648, and NS112779).
Conflict of Interest
The authors report no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
MG drafted the manuscript. TT edited the manuscript. Both have approved the revised version of the manuscript and agree to publish.
Rights and permissions
About this article
Cite this article
Gage, M.C., Thippeswamy, T. Inhibitors of Src Family Kinases, Inducible Nitric Oxide Synthase, and NADPH Oxidase as Potential CNS Drug Targets for Neurological Diseases. CNS Drugs 35, 1–20 (2021). https://doi.org/10.1007/s40263-020-00787-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00787-5