Abstract
Objective
The aim of the study was to compare the cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate, and intramuscular (IM) interferon (IFN)-β1a as first-line therapies in the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).
Methods
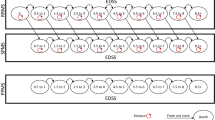
A Markov model was developed to evaluate the cost effectiveness of disease-modifying drugs (DMDs) from a US societal perspective. The time horizon in the base case was 5 years. The primary outcome was incremental net monetary benefit (INMB), and the secondary outcome was incremental cost-effectiveness ratio (ICER). The base case INMB willingness-to-pay (WTP) threshold was assumed to be US$150,000 per quality-adjusted life year (QALY), and the costs were in 2012 US dollars. One-way sensitivity analyses and probabilistic sensitivity analysis were conducted to test the robustness of the model results.
Results
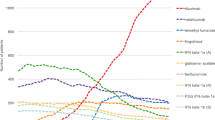
Dimethyl fumarate dominated all other therapies over the range of WTPs, from US$0 to US$180,000. Compared with IM IFN-β1a, at a WTP of US$150,000, INMBs were estimated at US$36,567, US$49,780, and US$80,611 for fingolimod, teriflunomide, and dimethyl fumarate, respectively. The ICER of fingolimod versus teriflunomide was US$3,201,672. One-way sensitivity analyses demonstrated the model results were sensitive to the acquisition costs of DMDs and the time horizon, but in most scenarios, cost-effectiveness rankings remained stable. Probabilistic sensitivity analysis showed that for more than 90 % of the simulations, dimethyl fumarate was the optimal therapy across all WTP values.
Conclusion
The three oral therapies were favored in the cost-effectiveness analysis. Of the four DMDs, dimethyl fumarate was a dominant therapy to manage RRMS. Apart from dimethyl fumarate, teriflunomide was the most cost-effective therapy compared with IM IFN-β1a, with an ICER of US$7,115.





Similar content being viewed by others
References
Stavnitser A, Patel N, Miller, A, et al. Impact of new oral therapies on multiple sclerosis cost and utilization trends. http://www.ajmc.com. Accessed 05 Aug 2013.
Lee S, Baxter DC, Limone B, et al. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ. 2012;15:1088–96.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303.
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107.
Hay JW. Evaluation and review of pharmacoeconomic models. Expert Opin Pharmacother. 2004;5:1867–80.
Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain. 2010;133:1914–29.
Drummond MF, Schulper MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. New York: Oxford University Press; 2007.
World Health Organization. Cost-effectiveness thresholds, http://www.who.int/choice/costs/CER_thresholds/en/. Accessed 19 Aug 2014.
The World Bank. GDP per capita, http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 01 June 2013.
Braithwaite SR, Meltzer D, King J, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life year decision rule? Med Care. 2008;46:349–56.
Villacorta R, Hay JW, Messali A. Cost effectiveness of moderate to severe psoriasis therapy with etanercept and ustekinumab in the United States. PharmacoEconomics. 2013;31:823–39.
Mehta D, Hay JW. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol Oncol. 2014;132:677–83.
Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States: a literature review and Markov Model. Neuro Oncol. 2013;15:1532–42.
Hay JW. Where’s the value in health care? Value Health. 2006;9:11–4.
National Multiple Sclerosis Society. Treatments, http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/treatments/index.aspx. Accessed 10 May 2013.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology. 1983;33:1444.
Kobelt G, Berg J, Atherley D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the USA. Neurology. 2006;66:1696–702.
Goodin DS, Cohen BA, O’Connor P, et al. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–73.
Castillo-Trivino T, Mowry EM, Gajofatto A, et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One. 2011;6:e16664.
Halpern R, Agarwal S, Borton L, et al. Adherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysis. Adv Ther. 2011;28:761–75.
Prosperini L, Gianni C, Leonardi L, et al. Escalation to natalizumab or switching among immunomodulators in relapsing multiple sclerosis. Mult Scler. 2012;18:64–71.
Tremlett H, Zhao Y, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler. 2008;14:314–24.
Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13:245–61.
Earnshaw SR, Graham J, Oleen-Burkey M, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy. 2009;7:91–108.
Jankovic SM, Kostic M, Radosavljevic M, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on data a Balkan country in socioeconomic transition. Vojnosanit Pregl. 2009;66:556–62.
Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I: clinical course and disability. Brain. 1989;112:133–46.
Kremenchutzky M, Rice GP, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129:584–94.
Scalfari A, Neuhaus A, Daumer M, et al. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 2013;70:214–22.
Guo S, Pelligra C, Thibault CSL, et al. Cost-effectiveness analyses in multiple sclerosis: a review of modelling approaches. Pharmacoeconomics. 2014;32:559–72.
Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value Health. 2012;15:843–50.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”): I. Validation of the method. Am J Med. 1982;73:883–8.
Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the “DEALE”): II. Use in medical decision-making. Am J Med. 1982;73:889–97.
Scalfari A, Neuhaus A, Daumer M, et al. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77:1246–52.
Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26:102–7.
Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–65.
Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler. 2003;9:311–9.
Torrance GW, Furlong W, Feeny D. Health utility estimation. Expert Rev Pharmacoecon Outcomes Res. 2002;2:99–108.
Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5:1–30.
Crayton H. Improved quality of life after therapy change to fingolimod. In: 27th Annual Meeting of the CMSC and the 5th Cooperative Meeting of the CMSC-ACTRIMS. Orlando, USA, 29 May-01 June 2013, Hackensack: CMSC.
Montalban X, Comi G, O’Connor P, et al. Oral fingolimod (FTY720) in relapsing multiple sclerosis: impact on health-related quality of life in a phase II study. Mult Scler. 2011;17:1341–50.
Rudick RA, Miller D, Hutchinson M, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–46.
O’Connor P, Briggs A, Carita P, et al. Impact on health-related quality of life of teriflunomide treatment by estimating utilities in patients with relapsing multiple sclerosis: results from TEMSO post hoc analysis. J Neurol. 2012;259:S107.
Kita M, Fox RJ, Phillips JT, et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: findings from the CONFIRM study. Mult Scler. 2014;20:253–7.
Kappos L, Gold R, Arnold DL. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: the DEFINE study. Mult Scler. 2014;20:243–52.
Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine: report of the panel on cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 250.
U.S. Department of Veterans Affairs. Drug pharmaceutical prices, http://www.pbm.va.gov/PharmaceuticalPrices.asp. Accessed 22 Aug 2013.
Oleen-Burkey M, Castelli-Haley J, Lage MJ, et al. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5:57–69.
Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8:1–2.
Briggs A, Schulper MJ, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–87.
O’Day K, Meyer K, Miller RM, et al. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14:617–27.
Pittock SJ, Mayr WT, McClelland RL, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004;62:601–6.
Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66:172–7.
Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15:543–55.
Acknowledgments
Xinke Zhang, Dr. Hay and Xiaoli Niu have no conflicts of interest to declare. No funding was received for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Hay, J.W. & Niu, X. Cost Effectiveness of Fingolimod, Teriflunomide, Dimethyl Fumarate and Intramuscular Interferon-β1a in Relapsing-Remitting Multiple Sclerosis. CNS Drugs 29, 71–81 (2015). https://doi.org/10.1007/s40263-014-0207-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0207-x