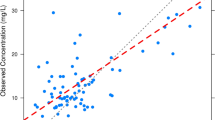
Abstract
Background and Objective
High variability in vancomycin exposure in neonates requires advanced individualized dosing regimens. Achieving steady-state trough concentration (C0) and steady-state area-under-curve (AUC0–24) targets is important to optimize treatment. The objective was to evaluate whether machine learning (ML) can be used to predict these treatment targets to calculate optimal individual dosing regimens under intermittent administration conditions.
Methods
C0 were retrieved from a large neonatal vancomycin dataset. Individual estimates of AUC0–24 were obtained from Bayesian post hoc estimation. Various ML algorithms were used for model building to C0 and AUC0–24. An external dataset was used for predictive performance evaluation.
Results
Before starting treatment, C0 can be predicted a priori using the Catboost-based C0-ML model combined with dosing regimen and nine covariates. External validation results showed a 42.5% improvement in prediction accuracy by using the ML model compared with the population pharmacokinetic model. The virtual trial showed that using the ML optimized dose; 80.3% of the virtual neonates achieved the pharmacodynamic target (C0 in the range of 10–20 mg/L), much higher than the international standard dose (37.7–61.5%). Once therapeutic drug monitoring (TDM) measurements (C0) in patients have been obtained, AUC0–24 can be further predicted using the Catboost-based AUC-ML model combined with C0 and nine covariates. External validation results showed that the AUC-ML model can achieve an prediction accuracy of 80.3%.
Conclusion
C0-based and AUC0–24-based ML models were developed accurately and precisely. These can be used for individual dose recommendations of vancomycin in neonates before treatment and dose revision after the first TDM result is obtained, respectively.






Similar content being viewed by others
References
Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42:S5–12.
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9. https://doi.org/10.1086/491712.
Rybak M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. https://doi.org/10.2146/ajhp080434.
Pham JT. Challenges of vancomycin dosing and therapeutic monitoring in neonates. J Pediatr Pharmacol Ther. 2020;25:476–84.
Wicha SG, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–41.
Allegaert K, Flint R, Smits A. Pharmacokinetic modelling and Bayesian estimation-assisted decision tools to optimize vancomycin dosage in neonates: only one piece of the puzzle. Expert Opin Drug Metab Toxicol. 2019;15:735–49. https://doi.org/10.1080/17425255.2019.1655540.
Roberts JA, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509. https://doi.org/10.1016/S1473-3099(14)70036-2.
McComb M, Bies R, Ramanathan M. Machine learning in pharmacometrics: opportunities and challenges. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.14801.
Jacqz-Aigrain E, et al. Population pharmacokinetic meta-analysis of individual data to design the first randomized efficacy trial of vancomycin in neonates and young infants. J Antimicrob Chemother. 2019;74:2128–38. https://doi.org/10.1093/jac/dkz158.
An SH, Lee EM, Kim JY, Gwak HS. Vancomycin pharmacokinetics in critically ill neonates receiving extracorporeal membrane oxygenation. Eur J Hosp Pharm. 2020;27:E25–9. https://doi.org/10.1136/ejhpharm-2018-001720.
Thomas CA, Picone A, Menon S, Willis BC. Empirical vancomycin dosing in pediatric patients with congenital heart disease and the impact of cardiopulmonary bypass on trough concentrations. Pharmacotherapy. 2018;37:1341–6.
Stone SB, Benner K, Utley A, MacLennan P, Coghill CH 3rd. Achieving vancomycin troughs within goal range in low birth weight neonates. J Pediatr Pharmacol Ther. 2021;26:56–61. https://doi.org/10.5863/1551-6776-26.1.56.
Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001;29:1189–232. https://doi.org/10.1214/aos/1013203451.
Dorogush AV, Ershov V, Gulin A. CatBoost: gradient boosting with categorical features support. 2018. https://arxiv.org/pdf/1810.11363.pdf. Accessed 3 Jun 2023.
Chen T, Tong H, Benesty M. xgboost: extreme gradient boosting. 2017. https://cran.microsoft.com/snapshot/2017-12-11/web/packages/xgboost/vignettes/xgboost.pdf. Accessed 3 Jun 2023.
Sheridan RP, Liaw A, Tudor M. Light gradient boosting machine as a regression method for quantitative structure–activity relationships. 2021. https://arxiv.org/pdf/2105.08626.pdf. Accessed 3 Jun 2023.
Hastie T, et al. The elements of statistical learning. Switzerland: Springer; 2009.
Basak D, Srimanta P, Patranbis DC. Support vector regression. Neural Inf Process Lett Rev. 2007;11:203–24.
Arik SO, Pfister T. TabNet: attentive interpretable tabular learning. 2019. https://arxiv.org/pdf/1908.07442.pdf. Accessed 3 Jun 2023.
Indyk, P. Approximate Nearest Neighbor: Towards Removing the Curse of Dimensionality. 1998. https://arxiv.org/pdf/1908.07442.pdf. Accessed 3 Jun 2023.
Ogami C, et al. An artificial neural network-pharmacokinetic model and its interpretation using Shapley additive explanations. CPT Pharmacometr Syst Pharmacol. 2021;10:760–8. https://doi.org/10.1002/psp4.12643.
Wan M, Walker S, Elaine M, Marion E, Lesley P, Leis JA. The impact of vancomycin trough concentrations on outcomes in non-deep seated infections: a retrospective cohort study. BMC Pharmacol Toxicol. 2018;19:47.
Kim J, et al. Determination of vancomycin pharmacokinetics in neonates to develop practical initial dosing recommendations. Antimicrob Agents Chemother. 2014;58:2830–40. https://doi.org/10.1128/AAC.01718-13.
Tseng S-H, et al. Evaluating the relationship between vancomycin trough concentration and 24-hour area under the concentration–time curve in neonates. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/AAC.01647-17.
Yao BF, et al. Predictive performance of pharmacokinetic model-based virtual trials of vancomycin in neonates: mathematics matches clinical observation. Clin Pharmacokinet. 2022;61:1027–38. https://doi.org/10.1007/s40262-022-01128-z.
Hughes JH, Tong DMH, Faldasz JD, Frymoyer A, Keizer RJ. Evaluation of neonatal and paediatric vancomycin pharmacokinetic models and the impact of maturation and serum creatinine covariates in a large multicentre data set. Clin Pharmacokinet. 2022. https://doi.org/10.1007/s40262-022-01185-4.
Sharland M. Manual of childhood infections: The Blue Book. Oxford: Oxford University Press; 2011.
Vancomycin hydrochloride for injection label from FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/210274s000lbl.pdf.
Custer JW. The Harriet lane handbook. USA: Johns Hopkins Hospital; 2020.
Ponthier L, et al. Optimization of vancomycin initial dose in term and preterm neonates by machine learning. Pharm Res. 2022;39:2497–506. https://doi.org/10.1007/s11095-022-03351-6.
Bououda M, et al. A machine learning approach to predict interdose vancomycin exposure. Pharm Res. 2022;39:721–31. https://doi.org/10.1007/s11095-022-03252-8.
Huang X, et al. An ensemble model for prediction of vancomycin trough concentrations in pediatric patients. Drug Des Devel Ther. 2021;15:1549–59. https://doi.org/10.2147/DDDT.S299037.
Nigo M, et al. PK-RNN-V E: a deep learning model approach to vancomycin therapeutic drug monitoring using electronic health record data. J Biomed Inf. 2022;133: 104166. https://doi.org/10.1016/j.jbi.2022.104166.
Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68:1243–55. https://doi.org/10.1007/s00228-012-1259-9.
Al-Jebawi Y, Karalic K, Shekhawat P, Mhanna MJ. The concomitant use of vancomycin and piperacillin-tazobactam is associated with acute kidney injury (AKI) in extremely low birth weight infants (ELBW). J Neonat Perinat Med. 2022;15(2):303–9.
Zhao W, et al. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob Agents Chemother. 2014;58:3191–9. https://doi.org/10.1128/AAC.02564-13.
Acknowledgments
We thank all of the patients who participated in this study and all of the participants and research staff in our hospital.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82173897), the Young Taishan Scholars Program of Shandong Province, and the Distinguished Young and Middle-aged Scholar of Shandong University.
Conflicts of Interest
Bo-Hao Tang, Jin-Yuan Zhang, Karel Allegaert, Guo-Xiang Hao, Bu-Fan Yao, Stephanie Leroux, Alison H. Thomson, Ze Yu, Fei Gao, Yi Zheng, Yue Zhou, Edmund V. Capparelli, Valerie Biran, Nicolas Simon, Bernd Meibohm, Yoke-Lin Lo, Remedios Marques, Jose-Esteban Peris, Irja Lutsar, Jumpei Saito, Evelyne Jacqz-Aigrain, John van den Anker, Yue-E Wu, and Wei Zhao declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
All the data were obtained from previous studies. These studies were approved by the institutional ethics committee.
Consent to Participate
All the data were obtained from previous studies. These studies were approved by the institutional ethics committee.
Consent for Publication
All participants received written informed consent in the previous studies.
Availability of Data and Material
Research data are not shared.
Code Availability
Research code available.
Authors' Contributions
Bo-Hao Tang wrote the manuscript; Wei Zhao designed the research; Karel Allegaert, Guo-Xiang Hao, Bu-Fan Yao, Stephanie Leroux, Alison Thomson, Yue-E Wu, Yi Zheng, Yue Zhou, Edmund V. Capparelli, Valerie Biran, Nicolas Simon, Bernd Meibohm, Yoke-Lin Lo, Remedios Marques, Jose-Esteban Peris, Irja Lutsar, Jumpei Saito, Evelyne Jacqz-Aigrain, and John van den Anker performed the research; Bo-Hao Tang and Jin-Yuan Zhang analyzed the data; and Ze Yu and Fei Gao contributed new reagents/analytical tools.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, BH., Zhang, JY., Allegaert, K. et al. Use of Machine Learning for Dosage Individualization of Vancomycin in Neonates. Clin Pharmacokinet 62, 1105–1116 (2023). https://doi.org/10.1007/s40262-023-01265-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01265-z