Abstract
Background and Objective
The risk of thrombotic complications in critical patients with COVID-19 remains extremely high, and multicenter trials failed to prove a survival benefit of escalated doses of low-molecular-weight heparins (nadroparin calcium) in this group. The aim of this study was to develop a pharmacokinetic model of nadroparin according to different stages of COVID-19 severity.
Methods
Blood samples were obtained from 43 patients with COVID-19 who received nadroparin and were treated with conventional oxygen therapy, mechanical ventilation, and extracorporeal membrane oxygenation. We recorded clinical, biochemical, and hemodynamic variables during 72 h of treatment. The analyzed data comprised 782 serum nadroparin concentrations and 219 anti-Xa levels. We conducted population nonlinear mixed-effects modeling (NONMEM) and performed Monte Carlo simulations of the probability of target attainment for reaching 0.2–0.5 IU/mL anti-Xa levels in study groups.
Results
We successfully developed a one-compartment model to describe the population pharmacokinetics of nadroparin in different stages of COVID-19. The absorption rate constant of nadroparin was 3.8 and 3.2 times lower, concentration clearance was 2.22 and 2.93 times higher, and anti-Xa clearance was 0.87 and 1.1 times higher in mechanically ventilated patients and the extracorporeal membrane oxygenation group compared with patients treated with conventional oxygen, respectively. The newly developed model indicated that 5.900 IU of nadroparin given subcutaneously twice daily in the mechanically ventilated patients led to a similar probability of target attainment of 90% as 5.900 IU of subcutaneous nadroparin given once daily in the group supplemented with conventional oxygen.
Conclusions
Different nadroparin dosing is required for patients undergoing mechanical ventilation and extracorporeal membrane oxygenation to achieve the same targets as those for non-critically ill patients.
Clinical Trial Registration
ClinicalTrials.gov identifier no. NCT05621915.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We developed a population pharmacokinetic model of nadroparin in three stages of COVID-19. |
The pharmacokinetics of nadroparin in patients with COVID-19 differs depending on the disease stage. |
The probability of target attainment analysis was performed to find the dose required to achieve thromboprophylaxis targets. |
1 Introduction
The risk of thrombotic complications in critically ill patients with COVID-19 remains extremely high and reaches 35–49% in the cohort treated in the intensive care unit (ICU), despite thromboprophylaxis [1, 2]. The risks and scale of pulmonary embolism, deep vein thrombosis, and cerebrovascular incidents increase proportionally to the severity of the disease and duration of ICU treatment [3, 4]. Addressing the aforementioned risks with accurate dosing of antithrombotic prophylaxis is of vital importance to the clinical society and medical policymakers [5].
Thrombotic prophylaxis in the ICU is most commonly exerted via subcutaneous (s.c.) administration of a fixed dose of low-molecular-weight heparin (LMWH) [6]. Several randomized clinical trials aimed to determine the benefit and risks of intermediate and therapeutic doses of LMWH in critically ill patients with COVID-19 [7,8,9]. The INSPIRATION Trial and REMAP-CAP investigators have found an association between intermediate and therapeutic dosing of LMWH and major bleeding complications in critically ill patients [7, 9]. In contrast, in moderately ill patients, a therapeutic dose of LMWH increased the probability of survival until hospital discharge at the cost of an increased bleeding rate [10]. The presented findings need urgent critical reappraisal [5]. Insight into the pharmacokinetics and pharmacodynamics of LMWH in different stages of COVID-19 could guide clinicians’ decision making at the bedside and enable precise and timely (once- or twice-daily) dosing of LMWH thromboprophylaxis [11].
According to the guidelines, in challenging patients, LMWH dosing may be monitored by the peak anti-Xa factor levels with prophylactic level targets between 0.2 and 0.5 IU/mL [12]. However, in several observational studies, the mean anti-Xa factor levels after a s.c. dose of 40 mg of enoxaparin in ICU patients with COVID-19 was significantly lower than in general ward patients, and 95% of ICU patients did not achieve the targeted anti-Xa factor levels [13]. Several studies confirmed that unmonitored fixed prophylactic doses of LMWH in the ICU may be given in a sub-therapeutic regimen [14, 15].
Population pharmacokinetics studies are among the methods used to identify and quantify sources of variability in drug concentrations, and are already extensively used to guide antibiotic therapy in the critically ill [16, 17]. Associations found between patient characteristics and differences in pharmacokinetics could facilitate point-of-care LMWH pharmacotherapy.
The primary aim of this study was to develop a population pharmacokinetic model of LMWH (nadroparin calcium) based on measured nadroparin concentrations and anti-Xa levels in different stages of COVID-19 severity. The secondary aims were to examine the relationship between patient characteristics and individual pharmacokinetic parameters and to provide simulations of reaching the anti-Xa target values after applying different dosing regimens in the study groups.
2 Methods
2.1 Study Design
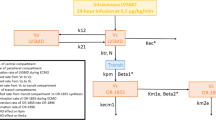
This prospective observational study was approved by the local ethics committee (consent number KE 0254/23/2021) and performed in accordance with the ethical standards of the Helsinki Declaration. The study was registered in ClinicalTrials.gov, study number NCT05621915. We included 43 consecutive patients treated in the Department of Anesthesiology and Intensive Care and the Department of Infectious Diseases of a tertiary academic hospital. All analyzed patients had been confirmed to have severe acute respiratory syndrome coronavirus 2 infection with a real-time reverse transcription-polymerase chain reaction or an antigen test. Oral consent was obtained from each conscious patient. In the case of unconscious patients or mechanically ventilated patients, the consent was waived by the ethics committee. Because of the observational nature of the study, no formal power calculation was performed. The sample size was based upon a “convenience samples,” that is, a reasonable number of subjects given the various constraints of the study, i.e., the inclusion/exclusion criteria, the timeline for enrollment, and the budget for the study. The first group (Group 1) consisted of 14 patients treated with conventional oxygen therapy only in the infectious diseases department. The second group (Group 2) consisted of 14 mechanically ventilated patients treated in the ICU, and the third group (Group 3) was formed by 15 patients supported with extracorporeal membrane oxygenation (ECMO) and mechanically ventilated in the ICU. We used surviving sepsis campaign guidelines for COVID-19 for the escalation of treatment in the study population [18]. Decisions to implement particular therapies were made according to the discretion of the physician.
2.2 Treatment Procedure
Thromboprophylaxis was exerted via s.c. injections of nadroparin calcium (Fraxiparine®; GlaxoSmithKline, Brentford, UK), usually in the lower abdominal area, in different treatment schedules. Patients in the first group, as well as in the second group, received a once-daily s.c. dose of 5700 IU of nadroparin. Three subjects from the second group received 5700 IU of nadroparin twice daily because of an elevated thrombotic risk assessed by the level of D-dimers and clots detected in an ultrasound examination of deep veins in the lower limbs. Patients in the third group, who were treated with ECMO and mechanically ventilated in the ICU, received s.c. 5700 IU of nadroparin twice daily. One patient in the ECMO group received s.c. 3800 IU of nadroparin twice daily because of a low body weight (56 kg) and the risk of postoperative bleeding.
2.3 Nadroparin Concentrations and Anti-Xa Measurements
Blood samples were collected on average four times between consecutive doses from each patient during 72 h of nadroparin administration and centrifuged at 3000 rpm for 15 min. The 29 patients receiving nadroparin twice daily were sampled a maximum 8 times a day and 24 times during the study. In the group that received nadroparin once daily, patients were sampled 4 times a day and 12 times during the whole study period. However, because of constrictions regarding the treatment regimen (surgery, diagnostic procedures, computed tomography scans) and the enzyme-linked immunosorbent assay (ELISA) concentration measurement method that was applied, some samples could not be taken precisely according to the study design or were excluded because of pre-diagnostic reasons (blood in the supernatant). Thus, out of 892 measurements that were planned, 782 nadroparin concentration measurements were included in the study. The supernatant plasma samples were then stored in plastic tubes at − 86 °C. The plasma LMWH concentrations were then determined by ELISA using the commercially available “human low molecular weight heparin ELISA kit” (MyBioSource, San Diego, CA, USA). Calculations were performed with a curve-fitting statistical software package (MyAssay; MyAssay Ltd, Brighton, UK). The nadroparin concentration in the activity units (9500 IU/1 mL) corresponds to 94.20 mg/mL in the mass concentration units. The conversion ratio was analytically determined based on measurements from five randomly selected vials.
Blood sampling for anti-Xa levels was performed twice daily (3.2% sodium citrate tubes (S-Monovette®; Sarstedt, Nümbrecht, Germany), with measurements taken at 4 h after a nadroparin dose (peak anti-Xa) and before the next nadroparin dose. Plasma anti-Xa levels were measured with HemosIL Liquid Anti-Xa (Instrumentation Laboratory, Bedford, MA, USA).
2.4 Clinical and Laboratory Variables
The following clinical and laboratory variables were recorded: partial pressure oxygen to fractional inspired oxygen ratio, albumin level, plasma urea, creatinine, international normalized ratio, prothrombin time, activated partial thromboplastin time, D-dimer, platelet, bilirubin, procalcitonin, C-reactive protein, partial pressure of oxygen, partial pressure of carbon dioxide, HCO3, lactate level, Sequential Organ Failure Assessment score, the presence of multi-organ failure, the Glasgow Coma Scale, the use of renal replacement therapy, diuresis, dobutamine administration, norepinephrine administration, cardiac index, Systemic Vascular Resistance Index, and extravascular lung water index during 72 h of the therapy by transpulmonary dilution (PICCO Pulsion; Getinge, Gothenburg, Sweden).
2.5 Population Pharmacokinetic Modeling
Population nonlinear mixed-effects modeling was conducted using NONMEM software (version 7.3; Icon Development Solutions, Ellicott City, MD, USA), a Fortran compiler (version 4.6.0), and Wings for NONMEM (version 741, http://wfn.sourceforge.net). First-order conditional estimation with the interaction method was employed throughout the model-building procedure. Data generated by NONMEM were processed and visualized using Matlab® (version 7.0; MathWorks, Inc., Natick, MA, USA).
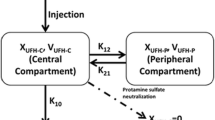
The minimum value of the NONMEM objective function, typical goodness-of-fit diagnostic plots, and the evaluation of the precision of pharmacokinetic parameters and variability estimates were used to discriminate between various models during the model-building process. A one-compartment disposition model with first-order absorption was used to describe plasma nadroparin concentrations and anti-Xa levels. The model is parametrized using apparent clearance, apparent volume of distribution, absorption rate constant, and proportionality constant between anti-factor Xa levels and nadroparin concentrations. The model’s performance was assessed by means of a visual predictive check. A nonparametric bootstrap was performed to evaluate the uncertainty of the final model’s parameters. The model building process and detailed methodology are described in the Electronic Supplementary Material (ESM).
2.6 Monte Carlo Simulations
The final model was used to simulate pharmacokinetic profiles expected for a subject for four dosing regimens (3800 IU and 5700 IU, given once or twice daily). The simulations were performed for the total duration of 4 days. The Monte Carlo method was used to generate individual pharmacokinetic parameters for n = 1000 subjects, based on the population pharmacokinetic parameters and random inter-individual effects obtained from the final model. This individual pharmacokinetic parameters were used to simulate the expected pharmacokinetic profiles. The outcomes of these simulations were represented as 50th (median) and (5–95th) percentiles.
The probability of target attainment (PTA) analysis was performed to translate the simulation into a clinically useful measure. The target was defined as an anti-Xa steady-state level 4 h post-dose being higher than 0.2 or 0.5 IU/mL. The probability representing the fraction of patients achieving that target was plotted for the nadroparin dose with a range from 2000 to 8000 IU.
3 Results
The analyzed data consisted of 782 nadroparin concentrations and 219 anti-Xa levels obtained from 43 patients. The raw concentration data of nadroparin and anti-Xa levels during 72 h of treatment (Fig. 1) and a summary of the patients’ characteristics in the three study groups are presented in Table 1.
Raw serum nadroparin concentrations (left column) and anti-Xa levels (right column) in particular patients of the three study groups with respect to different dosing regimens of nadroparin during 72 h of treatment. Straight lines connect points denoting nadroparin concentrations and anti-Xa levels for particular patients. conc. concentration, ECMO extracorporeal membrane oxygenation, h hours
3.1 Pharmacokinetic Model
A model-based population analysis was applied to the available pharmacokinetic data to obtain an integrated assessment of the pharmacokinetics of nadroparin after giving multiple doses to patients, to characterize various sources of variability observed in the data, and to explore the potential relationships between covariates and pharmacokinetic parameters. Based on visual inspection of the data, a one-compartment disposition model with first-order absorption was used to describe the available data. The anti-Xa levels were assumed to be proportional to the mass concentrations. Between-subject variability was estimated for apparent clearance, the absorption rate constant, and the proportionality constant. The variance for apparent volume of distribution was fixed to zero, as it tended to zero during the model-building process. Table 2 shows the parameter estimates of the final population’s pharmacokinetic model of nadroparin, along with their bootstrap estimates. Most pharmacokinetic parameters, between-subject variability (ω2), and residual error variances were estimated well, with the relative standard error lower than 50% for most parameters. The estimates of the model parameters fell very close to the median estimates obtained from the bootstrap, which proves the final model to be robust.
The typical values of the pharmacokinetic parameters were a priori assumed to be different for each group. Individual pharmacokinetic parameters stratified by study group are presented in Table 2 and the ESM. The inter-individual variability was low for apparent clearance in Group 1 (10.7%), high in Groups 2 and 3 (48.2%), intermediate for the apparent absorption rate constant (22.5%), and high for the proportionality constant (49.7%).
The apparent concentration clearance was considerably higher in the group of mechanically ventilated patients and patients supported with ECMO (2.55 L/h, and 3.37 L/h, respectively) in comparison to the group supplemented with conventional oxygen (1.15 L/h) (Table 2). Interestingly, the corresponding apparent anti-Xa clearance was more similar across the groups (1.36 L/h, 1.18 L/h, 1.50 L/h). We also observed a three-fold slower absorption rate of nadroparin in the critically ill COVID-19 population compared with patients with COVID-19 treated in regular wards with conventional oxygen therapy (Table 2).
3.2 Model Evaluation and Monte Carlo Simulations
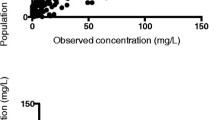
Basic goodness-of-fit plots of the final model are presented in Fig. 2. Individual and population predictions versus observed concentrations are relatively symmetrically distributed around the line of identity. The conditional weighted residuals versus time and versus individual predicted concentrations do not show any trend and are relatively evenly distributed around zero. There is only some small miscalibration for low anti-Xa levels that can be attributed to the presence of unaccounted below-quantification measurements and difficulty in building a more complex model owing to the limited number of anti-Xa level measurements. The visual predictive check plot indicates that both the central tendency of the data and the variability at a particular sampling time were captured well (ESM). The individual predicted concentration versus time profiles were very close to the experimental data as presented in the ESM.
Goodness-of-fit plots of the observed versus the population predicted nadroparin concentrations and anti-Xa levels and the observed versus the individual population predicted concentrations. Additionally, conditional weighted residuals (CWRES) are plotted versus individual predicted concentrations and time. Blue points denote nadroparin concentrations; green points denote anti-Xa levels. h hours
The relationship between the covariates and pharmacokinetic parameters was evaluated based on the ETA plots. The relationships for the selected covariates are shown in the ESM. The lack of any trend in these data indicates that these individual covariates do not account for the remaining unexplained between-subject variability of the pharmacokinetic parameters. It also indicates that we could not show the importance of these covariates for dose corrections.
We used an anti-Xa level of 0.2–0.5 IU/mL as the target value in the simulations provided in the present study to facilitate point-of-care nadroparin thromboprophylaxis in patients with COVID-19. In response to discussions of the urgent need to establish a treatment regimen for LMWH in critically ill patients, we calculated the PTA for different doses and administration frequencies in the three study groups (Fig. 3) [5, 11]. Moreover, based on our pharmacokinetic model, we included simulated anti-Xa levels for 72 h after a s.c. dose of 3800 or 5700 IU of nadroparin administered once or twice daily in three different stages of COVID-19 severity and related it to defined thromboprophylactic levels (Fig. 4).
Probability of achieving anti-Xa steady-state levels 4 h after a subcutaneous dose of nadroparin higher than 0.2 IU/mL (broken lines) or higher than 0.5 IU/mL (continuous lines) for different doses given once or twice daily in the three study groups. The horizontal line represents the probability of target attainment (PTA) of 0.9. Red lines represent the PTA in patients treated with conventional oxygen therapy, blue lines represent the PTA in patients treated with mechanical ventilation, and green lines represent the PTA in patients treated with mechanical ventilation and extracorporeal membrane oxygenation (ECMO). The vertical lines show the 3800 and 5700 IU doses of nadroparin
Simulated anti-Xa levels after different dosing regimens of subcutaneous nadroparin. Figures depict the simulated anti-Xa levels for different groups of patients after receiving subcutaneous (s.c.) nadroparin at a dose of 3800 or 5700 IU once or twice daily. The lines denote the mean and areas cover the 5–95th percentiles. The gray area corresponds to anti-Xa = 0.2–0.5 IU/mL. h hours
4 Discussion
To the best of our knowledge, this is the first study on the population pharmacokinetics of nadroparin in different stages of COVID-19 severity. In comparison to the studies published to date, the model was built based on both sequential ELISA measurements of concentrations of nadroparin and anti-Xa levels in the three stages of COVID-19 severity (Fig. 1) [35,36,37]. Moreover, the presented study is the first to establish population pharmacokinetics of nadroparin in the population treated with ECMO.
4.1 Population Pharmacokinetic Model
We used a one-compartment disposition model with first-order absorption to describe the population pharmacokinetics of nadroparin in the studied cohort, which corresponds to the previously published studies on the LMWH pharmacokinetics in the population of critically ill patients with COVID-19 [19,20,21]. In the literature, a one-compartment model was also used to describe nadroparin pharmacokinetics in children [22] and for rivaroxaban in patients with acute coronary syndrome [23]. In addition, a more complex two-compartment model was used to describe nadroparin in morbidly obese and non-obese patients [24], enoxaparin in infants, children, and adolescents during secondary thromboembolic prophylaxis [25], and unfractionated heparin pharmacokinetics during a cardiopulmonary bypass [26]. Nevertheless, the two-compartment model was not supported by our data. Nadroparin concentrations and anti-Xa levels were related, assuming a proportional relationship. This is a very simplistic assumption. However, a more complex model for anti-Xa was difficult to propose based on the data. In addition, between-subject variability for the proportionality constant was required to describe the data suggesting patients’ specific differences between concentrations and anti-Xa levels. This might be explained by the fact that the proportionality constant mostly reflects the fraction unbound of nadroparin (and also the lack of activity for some proportion of heparin molecules), as an anti-Xa assay measures unbound concentrations (enzymatic activity) and an ELISA measures total concentrations. Our observation regarding large inter-individual variation in the anti-Xa levels after nadroparin thromboprophylaxis in critically ill patients with COVID-19 confirmed earlier reports by Zijjeden et al. on dalteparin [20]. The authors attributed the presented finding to variations in both absorption and elimination.
According to the manufacturer, the bioavailability of nadroparin after a s.c. injection reaches 96%, and the estimated mean volume of distribution is 3.59 L. The mean elimination half-life ranges between 3.5 and 11.2 h, ensuring anti-Xa levels for 18 h after injection [27]. Worth noting for LMWHs, all pharmacokinetic parameters contribute to the maximum anti-Xa level attained 3–5 h after administration, for which a prophylactic range has been defined [28]. The elimination of nadroparin is mainly renal [27]. A small fraction of nadroparin is metabolized in the liver via desulfation and depolymerization.
4.2 Absorption
Only a few studies have assessed the s.c. absorption rate of LMWH in the population of patients treated with catecholamines or in the ICU. The absorption rate constant for nadroparin in the population of critically ill patients with COVID-19 (0.284 h−1) observed by Romano et al., and for enoxaparin (0.48 h−1) in the study published by Zufferey et al. corresponded to our results from the group treated with conventional oxygen [19, 20]. Our results may confirm earlier findings from the non-COVID-19 ICU population [29, 30]. In a previously published trial, the authors observed a decrease in anti-Xa levels after s.c. administration of fixed-dose nadroparin in patients supported with vasopressors [29]. Cihlar et al. found that norepinephrine dose and the peripheral blood perfusion evaluated by capillary refill time at the time of nadroparin application negatively correlated with peak levels of anti-Xa [30]. In contrast, van der Heijden et al. reported that huge variations in the absorption rate of dalteparin in critically ill patients with COVID-19 did not correlate with edema scores, capillary refill, or vasopressor use [21]. In the present study, we monitored the use of catecholamines, hemodynamic variables, and lactate levels in patients treated in the ICU (Table 2). However, we were not able to find a correlation between the pharmacokinetic variables of nadroparin and the latter.
Edema can also influence the uptake of LMWH. We have monitored the extent of edematic fluid in the body with the use of thermodilution in the group treated in the ICU. In 23 of 29 (79,04%) ICU patients, the extravascular lung water index was elevated above 14 mL/kg, which is the cut-off value for edema located in the pulmonary tissue. Moreover, two out of 29 ICU patients were treated with renal replacement therapy and ultrafiltration. Because other edema assessment methods, for example, bio-electrical impedance, are still not validated for the ICU population, we could not draw firm conclusions on the relationship of peripheral edema to nadroparin pharmacokinetics. The cause of the decreased absorption rate in critically ill patients with COVID-19 still merits further explanation (ESM).
4.3 Volume of Distribution
In the present study, the apparent volume of distribution of the central compartment in the group supplemented with conventional oxygen was higher than that reported by the manufacturer. Diepstraten et al. reported similar results (7 L) in a pharmacokinetic study of nadroparin in bariatric surgery in a group of non-obese patients [24]. In the studies on the critically ill COVID-19 population, Romano et al. observed that the volume of distribution of nadroparin was higher (11 L). Interestingly, in our study, in the group of ventilated patients and patients supported with ECMO, the volumes of distribution were much lower and close to the plasma volume (Table 2). Apparent volume of distribution was not related to actual body weight or any other variable. Based on mechanistic reasoning, one would expect an increased volume of distribution in the most critically ill patient group and in the ECMO group owing to potentially elevated capillary permeability and the extracorporeal circuit [31]. Nevertheless, our understanding of the relationship between volume of distribution and physiology is limited because the volume of distribution estimates are apparent and difficult to estimate precisely without nadroparin concentration data obtained after intravenous administration and a better understanding of absorption profiles.
4.4 Clearance
The apparent clearance of nadroparin in our study was slightly lower than the value estimated by Romano et al. in the population of critically ill patients with COVID-19 (2.23 L/h) [20]. In contrast, nadroparin clearance values observed in the non-COVID-19 aging population (0.60–0.8 L/h) presented by Mismetti et al. were much lower than those reported in patients with COVID-19 [32]. According to Romano et al. clearance of nadroparin in the critically ill patients was associated with an increase in inflammation parameters, while vasopressor and corticosteroid use significantly decreased nadroparin clearance by 25.1 and 22.5% [20]. According to Zufferey et al., enoxaparin clearance in mechanically ventilated patients with COVID-19 may result from augmented renal clearance, which is related to increased cardiac output and lower systemic vascular resistance [21]. We have monitored systemic vascular resistance, cardiac output, and catecholamine use in the cohort treated in the ICU and found no correlations between the latter (Table 1, ESM) [24, 32]. As only two patients from the ICU were supported with renal replacement therapy and kidney function was preserved in most patients, we cannot draw conclusions on the influence of impaired renal function on nadroparin clearance.
Interestingly, the differences in pharmacokinetic parameters (clearance and proportionality constant) suggest that different dosing strategies are required to achieve the same average concentrations or anti-Xa levels across the groups. It can be concluded that to achieve the same average nadroparin concentrations at a steady state, a typical ventilated patient (Groups 2 and 3) should receive about 2.2-fold and 2.9-fold higher doses than a typical patient from Group 1. However, to achieve the same average anti-Xa levels after nadroparin administration, a typical ventilated patient from Groups 2 and 3 should receive about 0.87-fold lower and 1.1-fold higher doses than typical patients from Group 1 (Table 2). Based on this reasoning, it seems that no adjustment is needed for the target defined by the average anti-Xa levels. Nevertheless, the target usually used (anti-Xa level 4 h post-dose) depends on all pharmacokinetic parameters of the model. More careful consideration is required to determine the dose leading to target attainment, for example, in the form of simulations or a PTA analysis.
4.5 Anti-Xa Guided Therapy
The LMWH prophylactic targets described in the literature range between 0.2 and 0.5 IU/mL [12]. However, anti-Xa levels assays do not correlate well with the drug’s effect, for example, activated clotting time, and may describe a drug’s pharmacokinetics rather than pharmacodynamics [33, 34].
Moreover, there are limited data on the relationship between the anti-Xa level and the effectiveness of thromboembolic prophylaxis. The prophylactic target range is not well validated in the literature [12]. Levine et al. observed a considerably higher incidence of thromboembolic events in patients where the anti-Xa levels 12 h after the s.c. administration of enoxaparin failed to reach the level of 0.1 IU/mL [35]. However, data provided in Levine et al.’s study after elective surgery may be incomparable to critically ill populations. According to Malinoski et al., failure to achieve an anti-Xa level of 0.1 IU/mL increased the risk for deep vein thrombosis in critically ill trauma patients, yet a precise prophylactic range was undefined [36]. Additionally, Droege et al. confirmed the correlation between the point-of-care prophylaxis and a decreased number of thromboembolic events. In the aforementioned study, administration of dalteparin was increased to twice daily if the anti-Xa level 12 h after s.c. administration of dalteparin was below 0.1 IU/mL, which resulted in a significant decrease in deep vein thrombosis incidence [37].
4.6 Dose Adjustment of Nadroparin
Based on the simulations provided in our study, it can be concluded that a single dose of 5990 IU of nadroparin in the group supplemented with passive oxygen led to a PTA of 90%, assuming the 0.2-IU/mL target (Fig. 3). For the group of mechanically ventilated patients, much higher doses are required to achieve the same target. For the twice-daily dosing regimen, a dose of 4770 or 5700, leads to a PTA of 90% for the 0.2 IU/mL target. The higher target of 0.5 IU/mL is achieved for about 25–30% of subjects in the case of the single daily regimen and 44–49% in the case of the twice-daily dosing regimen. There are conflicting data in the literature on the influence of doses of LMWHs on the peak anti-Xa level. van der Heijden et al. report that prophylactic dalteparin dosing per protocol would result in suboptimal dosing in 6% and supra-optimal dosing in 22% of critically ill patients with COVID-19 [21]. According to Zufferey et al., after s.c. 40 mg of enoxaparin, 64% of the patients would have peak concentrations within the defined prophylactic range, and 75% had 12-h concentrations above 0.1 IU/mL [19]. The authors suggest that 60 mg of enoxaparin daily could be optimal for thromboprophylaxis in ICU patients with COVID-19. In contrast, Romano et al. state that 5700 IU twice daily may be the most optimal dosing regimen, where 56.7% of patients may reach the predefined prophylactic target [36]. However, in the aforementioned study, different anti-Xa levels were defined as target values (0.3–0.7 IU/mL) [20]. Thus, in order to elucidate the issue of anti-Xa guided thromboprophylaxis, robust data on the relationship between clinical endpoints and prophylactic anti-Xa level, especially in critically ill patients, are urgently needed.
4.7 Limitations of the Study
The limitations of the study include a limited sample size. Because of the observational nature of the study, inclusion in the study group based on surviving sepsis campaign COVID-19 guidelines, and the discretion of the intensivist in patients’ dose adjustment, selection bias cannot be excluded. The duration of screening for nadroparin concentrations and anti-Xa levels was limited to only the first 72 h. We found discrepancies in the nadroparin concentrations and anti-Xa levels in the study groups, which merit further explanation. Anti-Xa levels were measured infrequently but according to the manufacturer’s recommendations. This is the first study using nadroparin concentrations and anti-Xa levels for the pharmacokinetic model-building process and thus needs validation in further studies. The influence of fluid and nutritional therapy could play a role in nadroparin pharmacokinetics.
5 Conclusions
Different nadroparin dosing strategies are required for patients undergoing mechanical ventilation and ECMO to achieve the same concentrations and anti-Xa levels as those of non-critically ill patients. Simulations provided with the use of population pharmacokinetic modeling could aid the decision-making process of clinicians at the bedside.
References
Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–72.
Shah A, Donovan K, McHugh A, Pandey M, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24(1):561.
Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–6.
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7.
Ten Cate H. Surviving Covid-19 with heparin? N Engl J Med. 2021;385(9):845–6. https://doi.org/10.1056/NEJMe2111151(Erratum in: N Engl J Med. 2021;385(11):1056).
Thachil J, Juffermans NP, Ranucci M, et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. 2020;18(9):2138–44.
REMAP-CAP Investigators; ACTIV-4a Investigators;ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777–89.
Sholzberg M, Tang GH, Rahhal H, RAPID Trial Investigators, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400.
INSPIRATION Investigators. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–30.
ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802.
Hofmaenner DA, Singer M. Challenging management dogma where evidence is non-existent, weak or outdated. Intensive Care Med. 2022;48(5):548–58.
Wei MY, Ward SM. The anti-factor Xa range for low molecular weight heparin thromboprophylaxis. Hematol Rep. 2015;7(4):5844.
Dutt T, Simcox D, Downey C, et al. Thromboprophylaxis in COVID-19: anti-FXa—the missing factor? Am J Respir Crit Care Med. 2020;202(3):455–7.
Piagnerelli M, Cauchie P, Vancutsem M, et al. Thromboprophylaxis in critically ill coronavirus disease 2019 patients. Crit Care Explor. 2020;2(8): e0177.
Stattin K, Lipcsey M, Andersson H, Pontén E, et al. Inadequate prophylactic effect of low-molecular weight heparin in critically ill COVID-19 patients. J Crit Care. 2020;60:249–52.
Borsuk-De Moor A, Sysiak-Sławecka J, Rypulak E, et al. Nonstationary pharmacokinetics of caspofungin in ICU patients. Antimicrob Agents Chemother. 2020;64(9):e00345-e420.
Charles B. Population pharmacokinetics: an overview. Aust Prescr. 2014;37:210–3.
Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–34.
Zufferey PJ, Dupont A, Lanoiselée J, Bauters A, Poissy J, Goutay J, et al. Pharmacokinetics of enoxaparin in COVID-19 critically ill patients. Thromb Res. 2021;205:120–7.
Romano LGR, Hunfeld NGM, Kruip MJHA, Endeman H, Preijers T. Population pharmacokinetics of nadroparin for thromboprophylaxis in COVID-19 intensive care unit patients. Br J Clin Pharmacol. 2022. https://doi.org/10.1111/bcp.15634.
van der Heijden CDCC, Ter Heine R, Kooistra EJ, Brüggemann RJ, Walburgh Schmidt JWJ, de Grouw EPLM, et al. Effects of dalteparin on anti-Xa activities cannot be predicted in critically ill COVID-19 patients. Br J Clin Pharmacol. 2022;88(6):2982–7.
Laporte S, Mismetti P, Piquet P, Doubine S, Touchot A, Decousus H. Population pharmacokinetic of nadroparin calcium (Fraxiparine) in children hospitalised for open heart surgery. Eur J Pharm Sci. 1999;8(2):119–25.
Xu XS, Moore K, Burton P, Stuyckens K, Mueck W, Rossenu S, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74(1):86–97.
Diepstraten J, Janssen EJ, Hackeng CM, van Dongen EP, et al. Population pharmacodynamic model for low molecular weight heparin nadroparin in morbidly obese and non-obese patients using anti-Xa levels as endpoint. Eur J Clin Pharmacol. 2015;71(1):25–34.
Trame MN, Mitchell L, Krümpel A, Male C, Hempel G, Nowak-Göttl U. Population pharmacokinetics of enoxaparin in infants, children and adolescents during secondary thromboembolic prophylaxis: a cohort study. J Thromb Haemost. 2010;8(9):1950–8.
Delavenne X, Ollier E, Chollet S, Sandri F, Lanoiselée J, Hodin S, et al. Pharmacokinetic/pharmacodynamic model for unfractionated heparin dosing during cardiopulmonary bypass. Br J Anaesth. 2017;118(5):705–12.
Nadroparin calcium injection. Canada product monograph (revised January 2019). https://pdf.hres.ca/dpd_pm/00049107.pdf. Accessed 28 Jun 2022.
Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–83.
Dörffler-Melly J, de Jonge E, Pont AC, Meijers J, Vroom MB, Büller HR, et al. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002;359(9309):849–50.
Cihlar R, Sramek V, Papiez A, Penka M, Suk P. Pharmacokinetic comparison of subcutaneous and intravenous nadroparin administration for thromboprophylaxis in critically ill patients on vasopressors. Pharmacology. 2020;105(1–2):73–8.
Cheng V, Abdul-Aziz MH, Roberts JA, Shekar K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(Suppl 5):S629–41.
Mismetti P, Laporte-Simitsidis S, Navarro C, Sié P, d’Azemar P, Necciari J, et al. Aging and venous thromboembolism influence the pharmacodynamics of the anti-factor Xa and anti-thrombin activities of a low molecular weight heparin (nadroparin). Thromb Haemost. 1998;79(6):1162–5.
Thomas O, Lybeck E, Strandberg K, Tynngård N, Schött U. Monitoring low molecular weight heparins at therapeutic levels: dose-responses of, and correlations and differences between aPTT, anti-factor Xa and thrombin generation assays. PLoS One. 2015;10(1): e0116835.
Ping’an P. MingzhaoQ comparison of anti-Xa factor assay and ACT for monitoring the anticoagulation effects of low-molecular-weight heparins in elderly patients. Heart. 2011;97:A188.
Levine MN, Planes A, Hirsh J, Goodyear M, Vochelle N, Gent M. The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparin low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb Haemost. 1989;62(3):940–4.
Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010;68(4):874–80.
Droege ME, Mueller EW, Besl KM, Lemmink JA, Kramer EA, Athota KP, et al. Effect of a dalteparin prophylaxis protocol using anti factor Xa concentrations on venous thromboembolism in high-risk trauma patients. J Trauma Acute Care Surg. 2014;76(2):450–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Paweł Piwowarczyk, Marta Szczukocka, Wojciech Cios, Paulina Okuńska, Grzegorz Raszewski, Michał Borys, Paweł Wiczling, and Mirosław Czuczwar have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was approved by the local ethics committee (consent number KE 0254/23/2021) and performed in accordance with the ethical standards of the Helsinki Declaration.
Consent to participate
Oral consent was obtained from each conscious patient. In the case of unconscious patients or mechanically ventilated patients, the consent was waived by the ethics committee.
Consent for publication
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
Methodology: PP, PW, MC, MB, MS, WC, PO, GR; formal analysis: PP, PW, MC, MB, MS, WC, PO, GR; investigation, data curation: PP, PW, MC, MB, MS, WC, PO, GR; writing: original draft preparation: PP, PW, MC, MB, MS, WC, PO, GR; writing: review and editing: PP, PW, MC, MB, MS, WC, PO, GR; supervision: MC; project administration: PP. All authors have read and agreed to the published version of the manuscript and to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Piwowarczyk, P., Szczukocka, M., Cios, W. et al. Population Pharmacokinetics and Probability of Target Attainment Analysis of Nadroparin in Different Stages of COVID-19. Clin Pharmacokinet 62, 835–847 (2023). https://doi.org/10.1007/s40262-023-01244-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01244-4