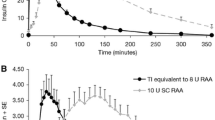
Abstract
Background
This study was performed to satisfy a US Food and Drug Administration post-marketing requirement to compare the dose responses for Technosphere® Insulin (TI; MannKind Corporation, Westlake Village, CA, USA) and subcutaneous insulin lispro (LIS) across a wide range of doses.
Objectives
This single-center, open-label, randomized, cross-over study defined the pharmacokinetic/pharmacodynamic curves for inhaled TI vs subcutaneous LIS in persons with type 1 diabetes mellitus.
Methods
Each volunteer received six treatments while undergoing euglycemic clamps: three doses of TI (10, 30 and 120 U) and LIS (8, 30, and 90 U). Primary endpoint was area under the glucose infusion rate vs time curve from start of treatment administration to end of clamp. Key secondary endpoints included readouts of insulin exposure and timing of pharmacokinetic/pharmacodynamic profiles.
Results
Insulin exposure was more than dose proportional, increasing with dose1.08 for LIS and dose1.35 for TI. Time to reach 10% of the maximum glucose infusion rate was 7 to 15 min for TI vs 21 to 38 min for LIS. End of effect was dose dependent for both treatments, ranging from 2 to 6 h (TI) and 5 to 10 h (LIS). Glucose infusion rate exhibited saturation for both treatments. Technosphere Insulin produced a lesser total effect per unit insulin than LIS due to its faster absorption and correspondingly shorter duration of exposure. The difference was large enough to require significantly different doses to achieve the same total effect.
Conclusions
Technosphere Insulin has a considerably faster onset and shorter duration of action than LIS. Consequently, the overall effect of TI is smaller than that of LIS and unit-for-unit dose conversion is not appropriate.
Clinical Trial Registration
ClinicalTrials.gov, NCT02470637; 12 June, 2015
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When the pharmacodynamic response is saturable, doubling a high dose (concentration) does not double the effect. |
Faster absorption of the drug produces higher concentrations and shorter durations, further reducing the effect. |
For insulin, the response to ultrarapid absorption, such as for intravenous bolus or inhaled Technosphere Insulin, is so different from subcutaneously injected regular human insulin and rapid-acting analogs that much higher doses are required for the same total effect. |
1 Introduction
Technosphere® Insulin (TI; MannKind Corporation, Westlake Village, CA, USA) is a dry-powder formulation of recombinant human insulin adsorbed onto Technosphere microparticles for oral inhalation and is approved for the treatment of both type 1 and type 2 diabetes mellitus in the USA and Brazil [1, 2]. In an earlier glucose clamp study comparing TI doses between 10 and 80 U against 15 U of subcutaneous (SC) regular human insulin (RHI), insulin exposure (area under the concentration vs time curve [AUC]) was dose proportional, but area under the glucose infusion rate (GIR) vs time curve (GIR AUC) from 0 to 4 h was less than dose proportional [3]. The US Food and Drug Administration was concerned that the pharmacodynamic (PD) effect of TI could be saturating at high doses and imposed a post-marketing requirement for a euglycemic clamp study comparing the pharmacokinetic (PK)/PD curves for TI and SC rapid-acting analog across a wide range of doses.
The current study was intended to identify the doses of TI and SC insulin in which saturation in GIR was evident. Aspects of the study design such as sample size (N = 30), sample population (persons with type 1 diabetes), and number of doses (three of each treatment) were negotiated with the Food and Drug Administration to ensure the study would meet the post-marketing requirement. Weaknesses of the previous study were addressed by (1) extending the GIR measurement until GIR returned to zero, (2) switching the reference treatment from RHI to SC insulin lispro (LIS), and (3) employing more than one dose of reference treatment. GIR AUC from 0 to 4 h from the TI study and GIR AUC from 0 to 12 h from a study of an SC rapid-acting analog [4] were fit to maximum effect (Emax) models as a function of dose. Doses corresponding to approximately 25%, 50%, and 80% of Emax were selected: 10, 30, and 120 U of TI and 8, 30, and 90 U of SC LIS.
In 1994, euglycemic clamp studies by Howey et al. demonstrated that the rate of insulin absorption affected its PD effect [5]. Intravenous (IV) RHI (10 U), with 100% bioavailability and nearly instantaneous absorption, produced insulin AUC that was 42% higher than SC LIS (10 U) or SC RHI (10 U) but a total PD effect (GIR AUC) that was 38% lower than either SC treatment. Faster insulin absorption was associated with higher peak concentrations and shorter durations of exposure. If GIR were proportional to plasma insulin concentration or concentration in the effect compartment, the same insulin AUC would produce the same GIR AUC over a shorter period. In Howey et al.’s study, GIR was saturable (exhibited an Emax behavior) such that a 30-fold range in peak insulin concentrations generated less than a two-fold range in maximum GIR (GIRmax).
Until recently, there was little interest in the effects of fast absorption rates on PD because there was no way to achieve them except by IV infusion. With the development of TI, however, much faster absorption is possible. For example, in a comparison of IV RHI, TI, and SC RHI, time to maximum concentration (Cmax) of insulin occurred 5 min after the start of administration of IV RHI, 13 min after administration of TI, and 121 min after administration of SC RHI [6].
In this paper, summary PK/PD parameters based on administered doses of TI and LIS are compared to address the concerns behind the post-marketing requirement. The effects of PK parameters (AUC from time 0 to the last measurable concentration [AUClast], Cmax of insulin, and mean residence time [MRT]) on PD response were also investigated.
2 Methods
2.1 Study Design and Population
This was a single-center, open-label, randomized, controlled, cross-over study in which volunteers received the three different doses of each treatment over a series of six euglycemic clamps (Electronic Supplementary Material [ESM]). The study was conducted in Neuss, Germany, after approval by the Independent Ethics Committee (Ärztekammer Nordrhein) and the Bundesinstitut für Arzneimittel und Medizinprodukte (Federal [German] Institute for Drugs and Medical Products). The trial was registered at ClinicalTrials.gov (NCT02470637).
Volunteers were eligible if they were between 18 and 65 years old, had a clinical diagnosis of type 1 diabetes for > 12 months, and were receiving a stable insulin regimen for ≥ 2 months before the study. Additional inclusion criteria were body mass index between 18.5 and 29.0 kg/m2 and both a forced expiratory volume in 1 second and a forced vital capacity ≥ 70% of predicted values in pulmonary function tests (ESM).
Technosphere Insulin was supplied as a dry powder for pulmonary delivery using the Gen2 (“Dreamboat”) inhaler [7]. Technosphere Insulin was administered as combinations of 10-U and 20-U cartridges totaling 10, 30, and 120 U of insulin. Single SC injections of 8, 30, and 90 U of LIS were administered by syringe horizontally 5 cm right and left of the umbilicus using a standardized skin-fold technique. Both treatments were administered under fasted conditions.
2.2 Euglycemic Clamp Procedure
Volunteers taking long-acting insulin switched to intermediate-acting or short-acting insulin products 48 h before dosing; those taking intermediate-acting insulin switched to short-acting insulin 24 h before dosing; and those receiving continuous insulin infusions discontinued pump therapy 6 h before dosing. The last allowed dose of prandial insulin was 12 h before dosing with TI or LIS, although small doses of short-acting analogs (≤ 6 U) were permitted up to 8 h before dosing.
The clamp procedure was performed using the ClampArt® device (Profil, Neuss, Germany). A 20% glucose solution was infused to keep the individual’s blood glucose at the determined target level of 100 mg/dL. A second infusion pump delivered 0.9% of sodium chloride solution to keep the line unobstructed. Heparin (heparin 10,000 U per 100 mL of saline) was infused into the double-lumen catheter at a rate of approximately 2 mL per hour to prevent blood clotting in the ClampArt double-lumen catheter. Blood glucose and GIR were continuously recorded during the clamp procedure.
Insulin glulisine, which exhibits less cross-reactivity with immunoasssays for human insulin than other analogs [8], was infused as needed to obtain a blood glucose concentration of 5.5 mmol/L (100 mg/dL); infusion of insulin glulisine was discontinued 20 min before treatment administration. When blood glucose levels had been stable for ≥ 1 h without any glucose infusion, TI or LIS was administered. Dosing occurred between 2.5 and 6.5 h after the start of the clamp procedure, and the clamps were planned for durations of 8–20 h post-dose (ESM). Clamps were terminated early if blood glucose values were ≥ 11.1 mmol/L (200 mg/dL) for 30 min in the absence of a glucose infusion. At the conclusion of the clamp, volunteers were disconnected from the device, received a meal with their usual prandial insulin therapy, and were advised on how to resume their prior insulin therapy.
2.3 Serum Insulin Concentrations
Serum concentrations of human insulin and LIS were measured using radioimmunoassays (Human Insulin-Specific RI; Millipore (Burlington, MA, USA), catalog number HI-14K; LisPro Insulin RIA; Millipore, catalog number LPI-16K) with a validated lower limit of quantification of 8 µU/mL. Blood samples for determination of serum insulin concentrations after administration of TI or LIS were collected on day 1 of each treatment period at 15 and 30 min pre-dose, directly pre-dose, and at specified timepoints for the duration of the clamp.
2.3.1 Endpoints
The primary PD endpoint was area under the GIR curve from start of treatment administration to end of clamp (GIR AUC). Key secondary PD endpoints included GIRmax, time to GIRmax (tGIRmax), time to onset of effect (tonset; estimated as the time required to reach 10% of GIRmax), and time to end of effect (tGIRend; taken to be the end of glucose infusion). Pharmacokinetic endpoints calculated from baseline-corrected insulin profiles included Cmax, time to Cmax, and AUClast.
Mean residence time, a standard PK metric, was also calculated for each treatment:
where AUC is area under the concentration vs time curve for insulin and AUMC is area under the concentration first-moment curve [9, 10]. Mean residence time quantifies the sum of average absorption time and average systemic residence time. Insulin is known to follow flip-flop kinetics, where the terminal decline in insulin concentration is governed by the rate of absorption, not systemic clearance [9, 10]. Once insulin is available systemically, its disposition is generally considered to be independent of administration route. Thus, differences in MRT reflect differences in mean absorption time, with lower MRT indicating faster absorption.
2.4 Safety
All volunteers exposed to TI or LIS were included in the safety population. Clinical laboratory tests, vital signs, physical examinations, adverse events (including serious adverse events), hypoglycemic events, and 12-lead electrocardiograms were assessed. As TI is an inhaled drug, pulmonary function testing and assessment of cough were also performed. Pulmonary function tests included spirometry (forced expiratory volume in 1 second and forced vital capacity) at the screening and end-of-study visits.
2.5 Statistical Analysis
Pharmacokinetic parameters Cmax, time to Cmax, and AUClast were computed from individual PK profiles after baseline subtraction. The lower limit of quantification (8 µU/mL) and baseline subtraction precluded an accurate calculation of the terminal half-life. Estimations of AUClast and AUMClast were calculated using the trapezoid rule and used to calculate MRT from Equation 1. The GIRmax and tGIRmax were obtained from individual GIR profiles fit by locally estimated scatterplot smoothing with a smoothing factor of 0.06; GIRmax was fit to
where Emax = Emax,LIS + TIΔ Emax,TI, X50 = X50,LIS + TIΔX50,TI, γ = 1+ΔγLIS+TIΔγTI, X = dose (D) or Cmax, and TI is the indicator variable for treatment (equal to 1 if TI was the treatment and 0 otherwise). All possible combinations of parameters were evaluated and the model with the lowest value of the Akaike information criterion (AIC) was selected.
The dependence of the “Y” variables AUClast, GIR AUC, MRT, and tGIRend on treatment and insulin dose or exposure was analyzed by linear regression on log-transformed variables with a maximal model of
where X = D or AUClast. The quadratic term was added to account for curvature. All combinations of β1–β5 were evaluated and the best model was identified based on AIC.
The GIR AUC was also fit to the maximal model
and the best combination of β1–β4 was identified by AIC.
3 Results
3.1 Patient Demographics
Thirty volunteers with type 1 diabetes participated in the study; 29 completed all six treatments and all profiles were analyzed. Mean age was 42.4 years, and 29 volunteers were male (96.7%) and White (96.7%). All volunteers had a body mass index of ≤ 29 kg/m2. Duration of type 1 diabetes averaged 22.5 years (range 3.5–46.5 years) and mean glycated hemoglobin was 7.4% (range 5.3–9.0%; ESM).
3.2 Safety
The most commonly reported treatment-emergent adverse event in the study was cough, reported by 10.0% (3/30) of volunteers for TI 30 U and 17.2% (5/29) of volunteers for TI 120 U; all reported incidents of cough were mild in intensity and occurred within 5 min of dosing. Symptomatic on-treatment hypoglycemia (defined as occurring during the time interval from treatment administration through to 11:59 p.m. the following day) was reported for two participants receiving TI 10 U, one receiving TI 30 U, and one participant receiving each of the three LIS doses. All the events occurred > 5.9 h after the end of the clamp and none of the events was considered to be related to treatment. No deaths, serious adverse events, or discontinuations due to adverse events were reported during the study.
3.3 Euglycemic Clamp Quality
Clamp quality was assessed by precision (coefficient of variation in blood concentrations during the clamp) and control deviation of blood glucose concentrations from the clamp target. The mean (standard deviation [SD]) values were less than 6.28% (1.47%) for precision and 2.21 (1.44) mg/dL for control deviation. The clamp quality in this study was comparable to that achieved in other studies with short-acting insulins [11, 12].
3.4 Pharmacokinetics and Pharmacodynamics
3.4.1 Analysis Related to Dose
Mean concentration profiles (Fig. 1a, b) illustrated increases in peak concentration and insulin exposure with increasing dose. The mean GIR curves (Fig. 1c, d) exhibited increases in GIR AUC and GIRmax that were clearly less than dose proportional. Peak insulin concentration, Cmax, occurred earlier for TI (14–18 min) than for LIS (73–95 min; Table 1). Mean tonset (estimated as the time required to reach 10% of GIRmax) for TI was 7–15 min vs 21–38 min for LIS; shorter times were associated with higher doses (Table 1). The duration of the glucose infusion increased with dose and ranged from 2 to 6.5 h for TI and from 5 to 10 h for LIS (Fig. 1c, d; Table 1).
Insulin exposure (AUClast) was proportional to D1.08 for LIS and D1.35 for TI (Fig. 2a) (see ESM for coefficients from the regression models). The data for LIS and TI did not exhibit a statistically significant curvature. Exponents greater than 1 indicated the bioavailability increased with increasing dose, consistent with linear absorption in parallel with Michaelis–Menten degradation in the depot. The geometric mean dose-normalized insulin exposure (AUClast/D) across doses was 165 min µU/mL/U for TI vs 883 min·µU/mL/U for LIS, yielding an overall relative bioavailability of 19%, with a range from 13% for the 10-U dose to 26% for the 120-U dose.
Dose dependence of key pharmacokinetic/pharmacodynamic summary parameters. a Insulin exposure (area under the concentration vs time curve from time 0 to the last measurable concentration [AUClast]), b insulin effect (glucose infusion rate area under the concentration vs time curve [GIR AUC]), c mean residence time (MRT), and d duration of effect (time to end of effect [taken to be the end of glucose infusion]) [tGIRend]. Solid curves are best fits calculated on log-transformed coordinates (lnY vs lnDose). LIS insulin lispro, TI Technosphere Insulin
The slopes of the GIR AUC best-fit curves decreased over the dose range studied, indicating progressively less incremental effect at higher doses (solid curves, Fig. 2b). On average, over the dose range studied (dotted lines, Fig. 2b), GIR AUC ~ D0.81 for LIS and ~ D1.20 for TI. The greater than dose-proportional increase in TI GIR AUC provided no evidence of saturation in total effect over the range of 10–120 U. The geometric mean value of the dose-normalized effect, GIR AUC/D, was 66.2 mg/kg/U for LIS vs 10.3 mg/kg/U for TI, yielding an overall relative bioeffect of 16%, with a dose-dependent range of 9–30%.
If insulin pharmacokinetics followed strict first-order kinetics, the MRT (Fig. 2c) would be constant and a plot of tGIRend vs lnD (Fig. 2d) would be a straight line with a slope inversely proportional to the terminal half-life of the effect [9]. Neither was true in this study. For both treatments, MRT increased with dose (MRTTI ~ D0.232 and MRTLIS ~ D0.139), indicating a slowing of absorption, clearance, or both. A power-law also fit the dose dependence of tGIRend (tGIRend,TI ~ D0.560, tGIRend,LIS ~D0.282), and the plot of tGIRend vs lnD was curved upward, indicating the terminal half-life of the effect became longer at higher doses.
Direct evidence showing that the dose range was large enough to demonstrate a saturable glucose effect was provided by GIRmax (Fig. 3). When fit as a function of dose (Fig. 3a), the best fit was obtained with γ = 1, a common value of Emax and a treatment-specific median effective dose (ED50). The parameter estimates (standard error) were: Emax = 17.3 (0.9) mg/kg/min, ED50,LIS = 18.2 (3.0) U, and ΔED50,TI = 45.7 (7.2) U; equivalently, ED50,TI = 63.8 (8.8) U. The best-fit parameters when modeled as a function of Cmax (Fig. 3b) included treatment-specific Emax with common values of half-maximal effective concentration and γ: Emax,LIS = 16.0 (0.9) mg/kg/min, Emax,TI = 11.5 (0.7) mg/kg/min, half-maximal effective concentration = 84.8 (10.1) µU/mL, and γ = 1.37 (0.19). The model based on Cmax was more likely with AIC = 851.4 vs 863.8 for the model based on dose.
Maximum effect (Emax) models for maximum glucose infusion rate (GIRmax). a GIRmax vs dose. Parameter estimates (standard error): Emax = 17.3 (0.9) mg/kg/min; median effective dose of insulin lispro (LIS) = 18.2 (3.0) U; median effective dose of Technosphere Insulin (TI) = 63.8 (8.8) U; γ = 1. b GIRmax vs maximum concentration (Cmax). Parameter estimates (standard error): Emax,LIS = 16.0 (0.9) mg/kg/min; Emax,TI = 11.5 (0.7) mg/kg/min; half-maximal effective concentration = 84.8 (10.1) µU/mL; γ = 1.37 (0.19). Solid curves represent best-fit curves. Dashed lines represent Emax
3.4.2 Analysis Related to PK Profiles
The relative bioavailability of TI (19%) and its relative bioeffect (16%) were similar, as were their variations with dose: 13–26% for relative bioavailability and 9–30% for relative bioeffect. These data suggested identical insulin exposures (AUClast) would produce similar insulin effects (GIR AUC), but that was not the case (Fig. 4a, b). At low insulin exposure, models estimated almost the same GIR AUC for both treatments, but the effects diverged at higher doses. The solid curves represented a fit with treatment and insulin exposure as independent variables (Eq. 3), while the dotted curves used insulin exposure and MRT as independent variables (Eq. 4). The cross-plots of observations vs predictions (Fig. 4c, d) illustrated how much of the treatment difference could be attributed to differences in PK profiles.
Pharmacodynamic effect as a function of insulin exposure. a Comparison of models based on treatment and area under the concentration vs time curve from time 0 to the last measurable concentration (AUClast) [solid curves] and AUClast and mean residence time (MRT) [dashed curves]—log-log plot, b comparison of models based on treatment and AUClast (solid curves) and AUClast and MRT (dashed curves)—linear coordinates, c scatter plot for model with treatment and AUClast, and d scatter plot for model with AUClast and MRT. AUC area under the concentration vs time curve, GIR glucose infusion rate, LIS insulin lispro, TI Technosphere Insulin
Insulin exposures for both treatments overlapped in the range of 4248–73,417 min µU/mL. Sets of n = 5 profiles for each treatment at the low and high ends of their common range permitted a direct comparison of pharmacokinetics and pharmacodynamics at comparable AUClast. The mean (SD) of five TI PK profiles at the low end of the overlap (6537 [638] min∙µU/mL) was compared with the mean (SD) of five LIS PK profiles with AUClast (6538 [65] min∙µU/mL; Fig. 5a). Similarly, the mean (SD) of five TI PK profiles at the high end of the overlap (AUClast = 61,489 [7327] min∙µU/mL) was compared with the mean (SD) of five LIS PK profiles with AUClast (61,371 [9082] min∙µU/mL; Fig. 5b). At low exposure (Fig. 5c), the higher Cmax for TI produced a higher mean GIRmax, but its total effect (551 mg/kg) was less than that of LIS (899 mg/kg) because of its shorter duration (shorter MRT). At high AUClast, GIR for TI was essentially at Emax over the first 2 h (Fig. 5d), further limiting GIR AUC (1654 mg/kg) relative to LIS (3946 mg/kg). Thus, the lesser effect of TI resulted from its faster absorption (lower MRT), which produced higher Cmax and drove GIR to near-maximal values at lower AUC. In addition to PK effects, Emax for TI was lower than that for LIS, further contributing to the difference between treatments. The comparison over 16 h is plotted at full scale in the ESM.
Comparison of pharmacokinetic (PK) and pharmacodynamic profiles at comparable insulin exposure. a Mean PK profiles (n = 5) with area under the concentration vs time curve from time 0 to the last measurable concentration (AUClast) = 6537 (638) min∙µU/mL (Technosphere Insulin [TI]) and 6538 (65) min∙µU/mL (insulin lispro [LIS]), b mean PK profiles (n = 5) with AUClast = 61,489 (7327) min∙µU/mL (TI) and 61,371 (9082) min∙µU/mL (LIS), c mean glucose infusion rate (GIR) profiles corresponding to the mean PK profiles in (a), and d mean GIR profiles corresponding to the mean PK profiles in (b)
4 Discussion
4.1 Study Results
The study was designed to identify doses of each treatment for which GIR AUC exhibited saturation. An Emax model fit to GIRmax yielded ED50,LIS = 18.2 U and ED50,TI = 68.8 U. As expected, saturation was evident in the curvature of GIR AUC vs D for both treatments, but overall, GIR AUC ~ D1.20 for TI because of the large increase in bioavailability. Rüppel et al. [3] simulated the previous study and concluded that (1) the apparent saturation in effect resulted from truncating the measurement of GIR at 4 h before it had returned to baseline, and (2) an Emax model of predicted GIR AUC vs D yielded ED50,RHI = 48 U and ED50,TI = 245 U. Although this study could not confirm the value of ED50,TI, there was no indication of saturation in the effect as a function of dose for TI at doses up to 120 U.
When the PD effect was related to insulin exposure rather than dose (Fig. 4), TI tended to produce a smaller effect for the same insulin AUC, and a regression analysis suggested that differences in absorption rate could explain much of the difference. Pharmacokinetic profiles with comparable insulin exposures but different MRTs were shown to generate significantly different GIR AUCs, with the faster-absorbing TI (lower MRT) producing less total effect than LIS. Maximum GIR for TI was also lower than that for LIS, although it was not clear that this was related to the PK profile. In Howey et al.’s comparison of 10 U of SC LIS to 10 U of IV RHI and 10 U of SC RHI [4], the MRTs for IV RHI, SC LIS, and SC RHI were 32, 101, and 235 min. At the same time, the mean effect per unit exposure ([GIR AUC]/AUC) relative to SC LIS was 43% for IV RHI and 99% for SC RHI. From the linear regression equations in the ESM, a 10-U dose of LIS would provide an insulin exposure of 8152 min µU/mL and a GIR AUC of 810 mg/kg; the same insulin exposure of TI, corresponding to 46 U of TI, would generate a GIR AUC of 563 mg/kg (69%). This value places TI between IV insulin and SC LIS, as does the estimated MRT of 44 min, vs 118 min for LIS.
The results from this study were consistent with Howey et al.’s and confirmed that extremely rapid delivery of insulin into systemic circulation produces less effect per unit of exposure than SC insulin, whether the rapid delivery is an IV bolus or ultra-rapid absorption from the lung [5, 6, 13]. Just as IV insulin and SC insulin were shown not to be interchangeable on a unit-for-unit basis, TI and SC insulin were not interchangeable for the same reason.
4.2 Clinical Use
4.2.1 Background Information on Dose Labeling
Technosphere Insulin cartridges are filled to contain 10, 20, or 30 U of insulin. At first glance, these seem like large doses, but the dose absorbed from the lungs is significantly lower because of delivery losses and degradation in the lungs. Therefore, during the Food and Drug Administration approval process, MannKind Corporation requested labeling that better reflected the effect of TI. The corresponding cartridges of Afrezza® (insulin human) inhalation powder (the commercial TI product) are therefore labeled as 4, 8, and 12 U based on the approximation that TI 10 U ~ SC insulin 4 U, which was used in a pivotal phase III trial in type 1 diabetes (AFFINITY 1; NCT01445951). This was a conservative conversion to set a starting dose for patients switching from SC insulin to TI, with the expectation that patients would titrate to achieve clinical effect. In AFFINITY 1, the average TI dose increased by 43%, suggesting that 10 U of TI ~ 2.8 U of SC insulin. The present study yielded an overall relative bioeffect of 16% and a range of 9–30%, suggesting that 10 U of TI (labeled as 4 U of Afrezza) ~ 2 U of SC insulin.
4.2.2 Clinical Dosing of TI
Given the difference between the labeled dose and its effect, a successful transition to TI will almost certainly require numerically higher doses to achieve comparable glycemic control. Even though the PD effect of TI is approximately half its labeled dose, and participants treated with TI in clinical trials were under-dosed, they experienced smaller postprandial glucose excursions than those receiving SC insulins for up to 90 min post-dose [14]. Longer periods of postprandial glucose control were demonstrated in a small pilot study employing continuous glucose monitors [15]. Volunteers with type 1 diabetes assigned to TI were switched according to the US package insert [1] and instructed to take supplemental doses 1 and/or 2 h after a meal if their postprandial glucose exceeded specified limits. Those participants who took 90% of the protocol-directed supplemental doses ended up taking twice the mean daily bolus dose as the control group taking insulin aspart (40.4 U of TI per day vs 20.6 U of aspart per day), while experiencing significantly more time in range and less time in hypoglycemia [15]. With its short MRT and correspondingly short duration of action, supplemental postprandial doses of TI can be taken without undue risk of hypoglycemia [16,17,18].
There is always uncertainty when translating results from euglycemic clamp studies into clinical practice. One limitation of this study is the rather homogeneous population, which comprised mostly male and White participants, rendering it difficult to transfer the results to other ethnic groups. However, published studies have not shown substantial differences in insulin effects between ethnic groups [19], and data from an as-yet unpublished bioequivalence study that comprised a more heterogeneous population (with 40% of the population reporting as Black or African American and mostly female participants) were consistent with the results reported here.
5 Conclusions
Inhaled TI represents an insulin option that offers a time-action profile intermediate between IV bolus and current SC insulin therapies [5]. Consequently, the PK/PD characteristics of TI include a faster onset, shorter duration, and lesser effect per unit dose than LIS or other SC injected insulins. These data suggest that transitioning to TI from SC insulin on a unit-for-unit basis is not appropriate for the same reason that IV bolus insulin and SC insulin are not dosed on an equal-unit basis.
References
Afrezza [package insert]. Westlake Village (CA): MannKind Corporation; 2018.
MannKind Corporation. Afrezza® (insulin human) inhalation powder approved in Brazil. In: Investors: press releases. 3 Jun 2019. https://investors.mannkindcorp.com/news-releases/news-release-details/afrezzar-insulin-human-inhalation-powder-approved-brazil. Accessed 16 Jun 2021.
Rüppel D, Dahmen R, Boss A, et al. A population dose-response model for inhaled Technosphere Insulin administered to healthy subjects. CPT Pharmacometrics Syst Pharmacol. 2017. https://doi.org/10.1002/psp4.12189.
Becker RHA, Frick AD, Nosek L, Heinemann L, Rave K. Dose-response relationship of insulin glulisine in subjects with type 1 diabetes. Diabetes Care. 2007. https://doi.org/10.2337/dc06-2114.
Howey D, Bowsher R, Brunelle R, Woodworth J. [Lys(B28), Pro(B29)]-human insulin: a rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396–402.
Steiner S, Pfützner A, Wilson B, Harzer O, Heinemann L, Rave K. Technosphere/Insulin: proof of concept study with a new insulin formulation for pulmonary delivery. Exp Clin Endocrinol Diabetes. 2002. https://doi.org/10.1055/s-2002-1998.
Smutney C, Grant M, Kinsey P. Device factors affecting pulmonary delivery of dry powders. Ther Deliv. 2013. https://doi.org/10.4155/tde.13.77.
Heurtault B, Reix N, Meyer N, et al. Extensive study of human insulin immunoassays: promises and pitfalls for insulin analogue detection and quantification. Clin Chem Lab Med. 2014. https://doi.org/10.1515/cclm-2013-0427.
Rowland M, Tozer T. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2011.
Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 4th ed. Stockholm: Swedish Pharmaceutical Press; 2000.
Heise T, Hövelmann U, Zijlstra E, Stender-Petersen K, Jacobsen J, Haahr H. A comparison of pharmacokinetic and pharmacodynamic properties between faster-acting insulin aspart and insulin aspart in elderly subjects with type 1 diabetes mellitus. Drugs Aging. 2017. https://doi.org/10.1007/s40266-016-0418-6.
Heise T, Stender-Petersen K, Hövelmann U, et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin Pharmacokinet. 2017. https://doi.org/10.1007/s40262-016-0473-5.
Heinemann L, Traut T, Heise T. Time-action profile of inhaled insulin. Diabet Med. 1997. https://doi.org/10.1002/(SICI)1096-9136(199701)14:1%3c63::AID-DIA298%3e3.0.CO;2-F.
McGill J, Weiss D, Grant M, Jones M, Kendall D, Hoogwerf B. Understanding inhaled Technosphere Insulin: results of an early randomized trial in type 1 diabetes mellitus. J Diabetes. 2020. https://doi.org/10.1111/1753-0407.13099.
Akturk H, Snell-Bergeon JK, Rewers A, et al. Improved postprandial glucose with inhaled Technosphere Insulin compared with insulin aspart in patients with type 1 diabetes on multiple daily injections: the STAT study. Diabetes Technol Ther. 2018. https://doi.org/10.1089/dia.2018.0200.
Bode B, McGill J, Lorber D, et al. Inhaled Technosphere Insulin compared with injected prandial insulin in type 1 diabetes: a randomized 24-week trial. Diabetes Care. 2015. https://doi.org/10.2337/dc15-0075.
Boss A, Petrucci R, Lorber D. Coverage of prandial insulin requirements by means of an ultra-rapid-acting inhaled insulin. J Diabetes Sci Technol. 2012. https://doi.org/10.1177/193229681200600406.
Seaquist ER, Blonde L, McGill JB, et al. Hypoglycaemia is reduced with use of inhaled Technosphere® Insulin relative to insulin aspart in type 1 diabetes mellitus. Diabet Med. 2020. https://doi.org/10.1111/dme.14202.
Hompesch M, Troupin B, Heise T, et al. Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006. https://doi.org/10.1111/j.1463-1326.2006.00643.x.
Acknowledgements
The authors thank Frank Pompilio, PharmD, and David Kendall, MD, for their contributions to this work. The authors also thank the participants in the study. Medical writing and editorial assistance were funded by MannKind Corporation, Westlake Village, CA, USA and provided under the direction of the authors by MedThink SciCom, Cary, NC, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Sanofi. Medical writing and editorial assistance were funded by MannKind Corporation.
Conflict of interest
MG is a paid employee of MannKind Corporation and may hold stock and stock options with the company and is listed as an inventor on several MannKind Corporation patents. TH has received research funds from Adocia, Afon Technology, AstraZeneca, Biocon, Boehringer Ingelheim, Eli Lilly, Gan Lee Pharmaceuticals, Johnson&Johnson, Julphar, Mylan, Nestlé, Neuraly, Nordic Bioscience, Novo Nordisk, Sanofi, and Zealand Pharma and has received speaker honoraria and travel grants from Eli Lilly and Novo Nordisk; he is also a member of advisory panels for Novo Nordisk and Valbiotis. RB is on several MannKind Corporation patents.
Ethics approval
The study was approved by the Independent Ethics Committee (Ärztekammer Nordrhein) and the Bundesinstitut für Arzneimittel und Medizinprodukte (Federal [German] Institute for Drugs and Medical Products).
Consent to participate
Informed consent was obtained prior to the conduct of any study-related procedures.
Consent for publication
Consent to publish anonymized data was obtained as part of the informed consent.
Availability of data and material
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
All authors contributed to the design, conduct/data collection, analysis, and writing of the manuscript and approve the manuscript for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Grant, M., Heise, T. & Baughman, R. Comparison of Pharmacokinetics and Pharmacodynamics of Inhaled Technosphere Insulin and Subcutaneous Insulin Lispro in the Treatment of Type 1 Diabetes Mellitus. Clin Pharmacokinet 61, 413–422 (2022). https://doi.org/10.1007/s40262-021-01084-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01084-0