Abstract
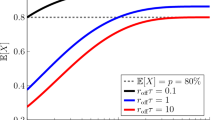
The medication adherence rate (A) is the proportion of prescribed drug doses consumed within a given time period. It is often assumed that there is an adherence rate threshold (A th) at or above which the therapeutic effect of the medication is maintained. Drug forgiveness (F) is the number of consecutive doses that can be missed while still maintaining a therapeutic effect. At a given value for A, the therapeutic effect of the drug will be continuously maintained if there is no possibility of >F missed doses. Hence, for a once-daily drug prescribed for N days, A th and F are related by the formula, A th = (N − F)/N. At adherence rates below A th the probability of maintaining the therapeutic effect is equal to the probability of there being no instances of >F consecutive missed doses. Since F is a function of the duration of the drug effect (D) and D varies depending on the specific drug’s pharmacokinetic/pharmacodynamic properties, there is no universal A th applicable to all drugs.


Similar content being viewed by others
References
Morrison A, Wertheimer AI. Compilation of quantitative overviews of studies of adherence. Drug Inf J. 2004;38(2):197–210.
Haynes RB, Sackett DL, Gibson ES, Taylor DW, Hackett BC, Roberts RS, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1(7972):1265–8.
Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74. doi:10.1002/pds.1230 (discussion 75–7).
Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44(9):1410–21. doi:10.1345/aph.1P150.
El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–79. doi:10.1111/bcp.12942.
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–310.
Global Adherence Interdisciplinary Network (GAIN). Defining adherence. In: Adherence to long-term therapies. Evidence for action. Geneva: World Health Organization; 2003.
Watanabe JH, Bounthavong M, Chen T. Revisiting the medication possession ratio threshold for adherence in lipid management. Curr Med Res Opin. 2013;29(3):175–80. doi:10.1185/03007995.2013.766164.
Urquhart J. Role of patient compliance in clinical pharmacokinetics: a review of recent research. Clin Pharmacokinet. 1994;27(3):202–15.
Boissel JP, Nony P. Using pharmacokinetic–pharmacodynamic relationships to predict the effect of poor compliance. Clin Pharmacokinet. 2002;41(1):1–6. doi:10.2165/00003088-200241010-00001.
Dartois V. Drug forgiveness and interpatient pharmacokinetic variability in tuberculosis. J Infect Dis. 2011;204(12):1827–9. doi:10.1093/infdis/jir662.
Urquhart J. The electronic medication event monitor: lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32(5):345–56. doi:10.2165/00003088-199732050-00001.
Pellock JM, Brittain ST. Use of computer simulations to test the concept of dose forgiveness in the era of extended-release (XR) drugs. Epilepsy Behav. 2016;55:21–3. doi:10.1016/j.yebeh.2015.11.029.
Ribaudo HJ, Haas DW, Tierney C, Kim RB, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42(3):401–7. doi:10.1086/499364.
Cohen CJ, Colson AE, Sheble-Hall AG, McLaughlin KA, Morse GD. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clin Trials. 2007;8(1):19–23. doi:10.1310/hct0801-19.
Dickinson L, Boffito M, Khoo SH, Schutz M, Aarons LJ, Pozniak AL, et al. Pharmacokinetic analysis to assess forgiveness of boosted saquinavir regimens for missed or late dosing. J Antimicrob Chemother. 2008;62(1):161–7. doi:10.1093/jac/dkn187.
Xiao Y, Miao H, Tang S, Wu H. Modeling antiretroviral drug responses for HIV-1 infected patients using differential equation models. Adv Drug Deliv Rev. 2013;65(7):940–53. doi:10.1016/j.addr.2013.04.005.
Faltaos DW, Urien S, Carreau V, Chauvenet M, Hulot JS, Giral P, et al. Use of an indirect effect model to describe the LDL cholesterol-lowering effect by statins in hypercholesterolaemic patients. Fundam Clin Pharmacol. 2006;20(3):321–30. doi:10.1111/j.1472-8206.2006.00404.x.
Kim J, Ahn BJ, Chae HS, Han S, Doh K, Choi J, et al. A population pharmacokinetic-pharmacodynamic model for simvastatin that predicts low-density lipoprotein-cholesterol reduction in patients with primary hyperlipidaemia. Basic Clin Pharmacol Toxicol. 2011;109(3):156–63. doi:10.1111/j.1742-7843.2011.00700.x.
Oh ES, Lee SH, Park MS, Park K, Chung JY. Modeling of the LDL cholesterol-lowering effect of atorvastatin in Korean dyslipidemic patients and non-patient volunteers. Int J Clin Pharmacol Ther. 2012;50(9):647–56.
Maclean R, Pfister M, Zhou Z, Roy A, Tuomari VA, Heifets M. Quantifying the impact of nonadherence patterns on exposure to oral immunosuppressants. Ther Clin Risk Manag. 2011;2011(7):149–56.
Morrison A, Stauffer ME, Kaufman AS. Defining medication adherence in individual patients. Patient Prefer Adher. 2015;9:893–7. doi:10.2147/PPA.S86249.
Fellows K, Rodriguez-Cruz V, Covelli J, Droopad A, Alexander S, Ramanathan M. A hybrid Markov chain-von Mises density model for the drug-dosing interval and drug holiday distributions. AAPS J. 2015;17(2):427–37. doi:10.1208/s12248-014-9713-5.
Acknowledgements
The authors thank Bob Greenwald for creating the Perl script described in ESM Sect. 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of interest
Alan Morrison, Melissa E. Stauffer, and Anna S. Kaufman declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morrison, A., Stauffer, M.E. & Kaufman, A.S. Relationship Between Adherence Rate Threshold and Drug ‘Forgiveness’. Clin Pharmacokinet 56, 1435–1440 (2017). https://doi.org/10.1007/s40262-017-0552-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0552-2