Abstract
Background and Objective
Pazopanib is a multi-targeted anticancer tyrosine kinase inhibitor. This study was conducted to develop a population pharmacokinetic (popPK) model describing the complex pharmacokinetics of pazopanib in cancer patients.
Methods
Pharmacokinetic data were available from 96 patients from three clinical studies. A multi-compartment model including (i) a complex absorption profile, (ii) the potential non-linear dose–concentration relationship and (iii) the potential long-term decrease in exposure was developed.
Results
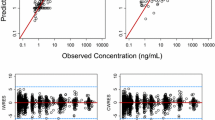
A two-compartment model best described pazopanib pharmacokinetics. The absorption phase was modelled by two first-order processes: 36 % (relative standard error [RSE] 34 %) of the administered dose was absorbed with a relatively fast rate (0.4 h−1 [RSE 31 %]); after a lag time of 1.0 h (RSE 6 %), the remaining dose was absorbed at a slower rate (0.1 h−1 [RSE 28 %]). The relative bioavailability (rF) at a dose of 200 mg was fixed to 1. With an increasing dose, the rF was strongly reduced, which was modelled with an E max (maximum effect) model (E max was fixed to 1, the dose at half of maximum effect was estimated as 480 mg [RSE 23 %]). Interestingly, the plasma exposure to pazopanib also decreased over time, modelled on rF with a maximum magnitude of 50 % (RSE 27 %) and a first-order decay constant of 0.15 day−1 (RSE 43 %). The inter-patient and intra-patient variability on rF were estimated as 36 % (RSE 16 %) and 75 % (RSE 22 %), respectively.
Conclusion
A popPK model for pazopanib was developed that illustrated the complex absorption process, the non-linear dose–concentration relationship, the high inter-patient and intra-patient variability, and the first-order decay of pazopanib concentration over time. The developed popPK model can be used in clinical practice to screen covariates and guide therapeutic drug monitoring.






Similar content being viewed by others
References
Votrient 200 mg film-coated tablets. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdf. Accessed 3 Sept 2015.
Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review(s) of Votrient. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022465s000_ClinPharmR.pdf. Accessed 13 Jan 2016.
van Leeuwen RWF, van Gelder T, Mathijssen RHJ, Jansman FGA. Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15(8):e315–26.
Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15(12):4220–7.
de Wit D, van Erp NP, den Hartigh J, Wolterbeek R, den Hollander-van Deursen M, Labots M, et al. Therapeutic drug monitoring to individualize the dosing of pazopanib: a pharmacokinetic feasibility study. Ther Drug Monit. 2014;37(3):331–8.
Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909–16.
Yu H, Steeghs N, Nijenhuis CM, Schellens JHM, Beijnen JH, Huitema ADR. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–25.
Yu H, Steeghs N, Kloth JSL, de Wit D, van Hasselt JGC, van Erp NP, et al. Integrated semi-physiological pharmacokinetic model for both sunitinib and its active metabolite SU12662. Br J Clin Pharmacol. 2015;79(5):809–19.
Kerbusch T, Huitema ADR, Ouwerkerk J, Keizer HJ, Mathôt RA, Schellens JHM, et al. Evaluation of the autoinduction of ifosfamide metabolism by a population pharmacokinetic approach using NONMEM. Br J Clin Pharmacol. 2000;49(6):555–61.
Kloth JSL, Klümpen H-J, Yu H, Eechoute K, Samer CF, Kam BLR, et al. Predictive value of CYP3A and ABCB1 phenotyping probes for the pharmacokinetics of sunitinib: the ClearSun study. Clin Pharmacokinet. 2014;53(3):261–9.
Diekstra MHM, Klümpen HJ, Lolkema MPJK, Yu H, Kloth JSL, Gelderblom H, et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin Pharmacol Ther. 2014;96(1):81–9.
Gotta V, Widmer N, Montemurro M, Leyvraz S, Haouala A, Decosterd LA, et al. Therapeutic drug monitoring of imatinib: Bayesian and alternative methods to predict trough levels. Clin Pharmacokinet. 2012;51(3):187–201.
Hamberg P, Mathijssen RHJ, de Bruijn P, Leonowens C, van der Biessen D, Eskens FA, et al. Impact of pazopanib on docetaxel exposure: results of a phase I combination study with two different docetaxel schedules. Cancer Chemother Pharmacol. 2014;75(2):365–71.
Hamberg P, Boers-Sonderen MJ, van der Graaf WTA, de Bruijn P, Suttle AB, Eskens FALM, et al. Pazopanib exposure decreases as a result of an ifosfamide-dependent drug-drug interaction: results of a phase I study. Br J Cancer. 2014;110(4):888–93.
Imbs D-C, Négrier S, Cassier P, Hollebecque A, Varga A, Blanc E, et al. Pharmacokinetics of pazopanib administered in combination with bevacizumab. Cancer Chemother Pharmacol. 2014;73(6):1189–96.
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Beal SL, Boeckman AJ, Sheiner LB, editors. NONMEM user guides. San Francisco: University of Califomia at San Francisco; 1988.
Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101(1):72–9.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008.
Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64.
The FDA Clinical Pharmacology and Biopharmaceutics Review(s) (application number 22-465) of Pazopanib (Votrient) (patient population Advanced Renal Cell Carcinoma). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022465s000_ClinPharmR.pdf. Accessed 6 July 2016.
Rowland M, Tozer TN. Chapter 7: absorption. Clinical pharmacokinetics and pharmacodynamics, 4th ed. Baltimore: Lippincott Williams & Wilkins; 2011. pp. 183–216.
Imbs D-C, Diéras V, Bachelot T, Campone M, Isambert N, Joly F, et al. Pharmacokinetic interaction between pazopanib and cisplatin regimen. Cancer Chemother Pharmacol. 2016;77(2):385–92.
Eechoute K, Fransson MN, Reyners AK, De Jong FA, Sparreboom A, Van Der Graaf WTA, et al. A long-term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res. 2012;18(20):5780–7.
Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30(5):2046–9.
Goh BC, Reddy NJ, Dandamudi UB, Laubscher KH, Peckham T, Hodge JP, et al. An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5 + 1 cocktail in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88(5):652–9.
Votrient: highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022465s-010S-012lbl.pdf. Accessed 3 Mar 2016.
Verheijen RB, Bins S, Mathijssen RH, Lolkema M, van Doorn L, Schellens JH, et al. Individualized pazopanib dosing: a prospective feasibility study in cancer patients. Clin Cancer Res. 2016. [Epub ahead of print]
Ahn JE, Birnbaum AK, Brundage RC. Inherent correlation between dose and clearance in therapeutic drug monitoring settings: possible misinterpretation in population pharmacokinetic analyses. J Pharmacokinet Pharmacodyn. 2005;32(5–6):703–18.
Acknowledgments
We would like to thank Prof. Dr. Hans Gelderblom who was the principal investigator of Study 1. We would like to thank Prof. Dr. Stefan Sleijfer and Dr. Paul Hamberg who were the principal investigators of Studies 2 and 3. In addition, we would like to thank Remy Verheijen for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used.
Conflict of interest
Huixin Yu, Nielka van Erp, Sander Bins, Ron Mathijssen, Jan Schellens, Jos Beijnen, Neeltje Steeghs and Alwin Huitema declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., van Erp, N., Bins, S. et al. Development of a Pharmacokinetic Model to Describe the Complex Pharmacokinetics of Pazopanib in Cancer Patients. Clin Pharmacokinet 56, 293–303 (2017). https://doi.org/10.1007/s40262-016-0443-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0443-y