Abstract
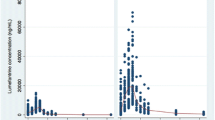
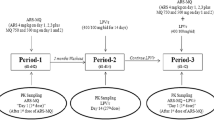
Management of HIV and malaria co-infection is challenging due to potential drug–drug interactions between antimalarial and HIV-antiviral drugs. Little is known of the clinical significance of these drug interactions, and this review provides a comprehensive summary and critical evaluation of the literature. Specifically, drug interactions between WHO-recommended artemisinin combination therapies (ACT) and HIV-antivirals are discussed. An extensive literature search produced eight articles detailing n = 44 individual pharmacokinetic interactions. Only data pertaining to artemether–lumefantrine and two other artesunate combinations are available, but most of the interactions are characterized on at least two occasions by two different groups. Overall, protease inhibitors (PIs) tended to increase the exposure of lumefantrine and decrease the exposures of artemether and dihydroartemisinin, a pharmacologically active metabolite of artemether. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) tended to decrease the exposures of artemether, dihydroartemisinin, and lumefantrine when co-administered with artemether–lumefantrine. Fewer studies characterized the effects of PIs or NNRTIs on artesunate combinations, but nevirapine increased artesunate exposure and ritonavir decreased dihydroartemisinin exposure. On the other hand, artemether–lumefantrine or artesunate combinations had little effect on the pharmacokinetics of HIV-antivirals, with the exception of decreased nevirapine exposure from artemether–lumefantrine or increased ritonavir exposure from pyronaridine/artesunate co-administration. In general, pharmacokinetic interactions can be explained by the metabolic properties of the co-administered drugs. Despite several limitations to the studies, these data do provide valuable insights into the potential pharmacokinetic perturbations, and the consistently marked elevation or reduction in ACT exposure in some cases cannot be overlooked.
Similar content being viewed by others
References
World Health Organization. Guidelines for the treatment of malaria. Second edition. March 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. Accessed 14 Jul 2013.
Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS epidemic update. December 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf. Accessed 14 Jul 2013.
German P, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47:91–102.
World Health Organization. Malaria and HIV and their implications for public policy, report of a technical consultation, Geneva, Switzerland, 23–25 June 2004. http://www.who.int/hiv/pub/prev_care/malariahiv.pdf. Accessed 14 Jul 2013.
Chalwe V, Van Geertruyden JP, Mukwamataba D, et al. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerg Infect Dis. 2009;15:749–55.
Grimwade K, French N, Mbatha DD, et al. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–54.
Ayisi JG, van Eijk AM, ter Kuile FO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:585–94.
Bloland PB, Wirima JJ, Steketee RW, et al. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS. 1995;9:721–6.
Van Geertruyden JP, Mulenga M, Mwananyanda L, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–25.
Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–40.
Khoo S, Back D, Winstanley P. The potential for interactions between antimalarial and antiretroviral drugs. AIDS. 2005;19:995–1005.
The Department for Health and Human Services [DHHS] guidelines Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; Feb 2013. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 14 Jul 2013.
Byakika-Kibwika P, Lamorde M, Mayanja-Kizza H, et al. Update on the efficacy, effectiveness and safety of artemether–lumefantrine combination therapy for treatment of uncomplicated malaria. Ther Clin Risk Manag. 2010;6:11–20.
Giao PT, de Vries PJ. Pharmacokinetic interactions of antimalarial agents. Clin Pharmacokinet. 2001;40:343–73.
Medhi B, Patyar S, Rao RS, et al. Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacology. 2009;84:323–32.
Nsanzabana C, Rosenthal P. In vitro activity of antiretroviral drugs against Plasmodium falciparum. Antimicrob Agents Chemother. 2011;55:5073–7.
German P, Parikh S, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–9.
Byakika-Kibwika P, Lamorde M, Okaba-Kayom V, et al. Lopinavir/ritonavir significantly influences pharmacokinetic exposure of artemether/lumefantrine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67:1217–23.
Kakuda T, Demasi R, van Delft Y, et al. Pharmacokinetic interaction between etravirine or darunavir/ritonavir and artemether/lumefantrine in healthy volunteers: a two-panel, two-way, two-period, randomized trial. HIV Med. 2013;14:421–9.
Wyen C, Fuhr U, Frank D, et al. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther. 2008;84:75–82.
McKeage K, Perry CM, Keam SJ. Darunavir: a review of its use in the management of HIV infection in adults. Drugs. 2009;69:477–503.
Lefevre G, Carpenter P, Souppart C, et al. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether–lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br J Clin Pharmacol. 2002;54:485–92.
van Agtmael MA, Cheng-Qi S, Qing JX, et al. Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int J Antimicrob Agents. 1999;12:151–8.
Yeh RF, Gaver VE, Patterson KB, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60.
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59.
Ilett KF, Ethell BT, Maggs JL, et al. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos. 2002;30:1005–12.
Zhang D, Chando TJ, Everett DW, et al. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–39.
Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether–lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–7.
Byakika-Kibwika P, Lamorde M, Lwabi P, et al. Cardiac conduction safety during coadministration of artemether–lumefantrine and lopinavir/ritonavir in HIV-infected Ugandan adults. Chemother Res Pract. 2011;2011:1–4.
Byakika-Kibwika P, Lamorde M, Mayito J, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;69:2213–21.
Huang L, Parikh S, Rosenthal PJ, et al. Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether–lumefantrine in healthy volunteers. J Acquir Immune Defic Syndr. 2012;61:310–6.
Kredo T, Mauff K, Van der Walt JS, et al. Interaction between artemether–lumefantrine and nevirapine-based antiretroviral therapy in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5616–23.
Hariparsad N, Nallani SC, Sane RS, et al. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44:1273–81.
Faucette SR, Zhang TC, Moore R, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80.
Yanakakis LJ, Bumpus NN. Biotransformation of the antiretroviral drug etravirine: metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab Dispos. 2012;40:803–14.
Lamorde M, Byakika-Kibwika P, Mayito J, et al. Lower artemether, dihydroartemisinin and lumefantrine concentrations during rifampicin-based tuberculosis treatment. AIDS. 2013;27:961–5.
Fehintola FA, Scarsi KK, Ma Q, et al. Nevirapine-based antiretroviral therapy impacts artesunate and dihydroartemisinin disposition in HIV-infected Nigerian adults. AIDS Res Treat. 2012;2012:1–6.
Morris CA, Lopez-Lazaro L, Jung D, et al. Drug–drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. Am J Trop Med Hyg. 2012;86:489–95.
Li XQ, Bjorkman A, Andersson TB, et al. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol. 2003;59:429–42.
Lamson M, MacGregor T, Riska P, et al. Nevirapine induces both CYP3A4 and CYP2B6 metabolic pathways. Clin Pharmacol Ther. 1999;65:137.
Aarnoutse RE, Kleinnijenhuis J, Koopmans PP, et al. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin Pharmacol Ther. 2005;78:664–74.
Croft SL, Duparc S, Arbe-Barnes SJ, et al. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012;11:270–98.
Teja-Isavadharm P, Watt G, Eamsila C, et al. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:717–21.
Dickinson L, Khoo S, Back D, et al. Differences in the pharmacokinetics of protease inhibitors between healthy volunteers and HIV-infected persons. Curr Opin HIV AIDS. 2008;3:296–305.
Acknowledgments
No sources of funding were used in the preparation of this review. Tony KL Kiang, Kyle J Wilby and Mary HH Ensom have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiang, T.K.L., Wilby, K.J. & Ensom, M.H.H. Clinical Pharmacokinetic Drug Interactions Associated with Artemisinin Derivatives and HIV-Antivirals. Clin Pharmacokinet 53, 141–153 (2014). https://doi.org/10.1007/s40262-013-0110-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0110-5