Abstract
Background and Objective
Acute lymphoblastic leukemia (ALL) is an acute, rapidly progressing and life-threatening form of cancer involving immature lymphocytes called lymphoblasts. ALL is the most common subtype of leukemia in children and adolescents. The aim of the present study was to assess the cost-utility of pegaspargase versus l-asparaginase, both followed by Erwinase in the therapy sequence, as a treatment option for pediatric, adolescent, and adult patients with ALL in Greece.
Methods
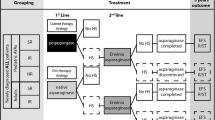
A published cost-utility model comprising a decision tree and a state-transition Markov model was adapted from a public payer perspective to compare a pegaspargase treatment sequence with an l-asparaginase sequence. Efficacy and safety data, as well as utility values, were extracted from the published literature. Direct costs pertaining to drug acquisition, administration, and management of hypersensitivity were considered in the analysis (€2020). Model-extrapolated outcomes included quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICER). All future outcomes were discounted at 3.5% per annum. Sensitivity analyses were used to explore the impact of changing input data.
Results
The analysis showed that the pegaspargase sequence was estimated to produce 0.05 additional QALYs (18.12 vs. 18.07) and lower cost of − €1698 compared with l-asparaginase, indicating that the pegaspargase sequence was a dominant treatment strategy (improved outcomes with reduced costs) compared with l-asparaginase. Deterministic sensitivity analysis confirmed the cost-effective profile of pegaspargase. At the defined willingness-to-pay threshold of €54,000/QALY gained, probabilistic sensitivity analysis showed that pegaspargase had a 100% probability of being cost effective relative to the l-asparaginase sequence.
Conclusion
The pegaspargase sequence was found to be less costly and more effective (in terms of QALYs) in relation to the l-asparaginase sequence, representing a dominant strategic option for Greek public payers in ALL.



Similar content being viewed by others
Change history
29 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40261-022-01236-5
References
Medawar CV, Mosegui GBG, Vianna CMM, et al. PEG-asparaginase and native Escherichia coli l-asparaginase in acute lymphoblastic leukemia in children and adolescents: a systematic review. Hematol Transfus Cell Ther. 2020;42(1):54–61. https://doi.org/10.1016/j.htct.2019.01.013.
Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v69–82. https://doi.org/10.1093/annonc/mdw025.
Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–9.
Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet. 2005;44(4):367–93. https://doi.org/10.2165/00003088-200544040-00003.
Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. https://doi.org/10.1182/blood-2006-06-027714.
Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood. 2001;97(5):1211–8. https://doi.org/10.1182/blood.v97.5.1211.
Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. https://doi.org/10.1056/NEJMra052603.
Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–94.
Zeidan A, Wang ES, Wetzler M. Pegasparaginase: where do we stand? Expert Opin Biol Ther. 2009;9(1):111–9. https://doi.org/10.1517/14712590802586058.
Angiolillo AL, Schore RJ, Devidas M, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli l-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol. 2014;32(34):3874–82.
European Medicines Agency (EMA). Available at: www.ema.europa.eu/en/medicines/human/EPAR/oncaspar. Accessed 30 June 2020.
Kurtzberg J, Asselin B, Bernstein M, et al. Polyethylene glycol-conjugated l-asparaginase versus native l-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–6. https://doi.org/10.1097/MPH.0b013e31822d4d4e.
Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–18. https://doi.org/10.1016/S1470-2045(14)70243-8.P.
Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. https://doi.org/10.1016/S1470-2045(12)70600-9.
Haithcox S, Ramnes CR, Lee H, et al. The impact of frequent injections for hematopoietic growth factor support on patients receiving chemotherapy: an observational study. BMC Nurs. 2003;2:2.
Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli l-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677–90. https://doi.org/10.1016/s1470-2045(15)00363-0.
Hu X, Wildman K, Basu S, et al. The cost-effectiveness of pegaspargase versus native asparaginase for first-line treatment of acute lymphoblastic leukaemia: a UK-based cost-utility analysis. Health Econ Rev. 2019;9(1):40.
Gourzoulidis G, Maniadakis N, Petrakis D, et al. Economic evaluation of trifluridine and tipiracil hydrochloride in the treatment of metastatic colorectal cancer in Greece. J Comp Effect Res. 2019;8:133–42. https://doi.org/10.2217/cer-2018-0076.
Vellopoulou K, Stefanou G, Tzanetakos C, et al. Cost-effectiveness of tofacitinib for the treatment of moderate to severe active ulcerative colitis in Greece. Eur J Gastroenterol Hepatol. 2021;33:325–33. https://doi.org/10.1097/meg.0000000000001916.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal. 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/involvement-and-participation. Accessed 1 Feb 2020.
European Network for Health Technology Assessment (EUnetHTA). Pharmacoeconomic guidelines around the world. May 2015. Available at: https://www.eunethta.eu/wp-content/uploads/2018/03/Methods_for_health_economic_evaluations.pdf. Accessed 10 Jan 2021.
Burke MJ, Devidas M, Maloney K, et al. Severe pegaspargase hypersensitivity reaction rates (grade ≥3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children’s oncology group (COG) clinical trials. Leukemia Lymphoma. 2018;59:1624–33.
Pieters R, Hunger SP, Boos J, et al. l-Asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011;117:238–49. https://doi.org/10.1002/cncr.25489.
Vrooman LM, Kirov II, Dreyer ZE, et al. Activity and toxicity of intravenous erwinia asparaginase following allergy to E coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63(2):228–33. https://doi.org/10.1002/pbc.25757.
World Health Organization (WHO). Greece life tables. Available at: http://apps.who.int/gho/data/?theme=main&vid=60640. Accessed 30 June 2020.
Graham ML. Pegaspargase: a review of clinical studies. Adv Drug Deliv Rev. 2003;55:1293–302. https://doi.org/10.1016/S0169-409X(03)00110-8.
Müller HJ, Boos J. Use of l-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113. https://doi.org/10.1016/S1040-8428(98)00015-8.
Burke MJ, Devidas M, Maloney K, et al. Severe pegaspargase hypersensitivity reaction rates (grade ≥3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children’s oncology group (COG) clinical trials. Leukemia lymphoma. 2018;59(7):1624–33. https://doi.org/10.1080/10428194.2017.1397658.
Pieters R, Hunger SP, Boos J, et al. l-Asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011;117(2):238–49. https://doi.org/10.1002/cncr.25489.
Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Available at: https://link.springer.com/chapter/10.1007/978-94-007-7596-1_3. 2014. Accessed 30 June 2020.
Furlong W, Rae C, Feeny D, et al. Health-related quality of life among children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:717–24.
National Institute for Health and Clinical Excellence (NICE). NICE clinical guideline 134. Anaphylaxis. Available at: http://www.nice.org.uk/guidance/cg134/evidence/anaphylaxis-full-guideline-184946941. Accessed 1 Feb 2020.
Greek Ministry of Health. Drug price bulletin 2020. Available at: https://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn. Accessed 30 June 2020.
Pharmaceutical Research and Technology company (I.F.E.T.). List of medicines. Available at: https://www.ifet.gr/en/. Accessed 30 June 2020.
Greek Ministry of Health. DRG code: Σ22X. Diagnostic related groups. 2020. Available at: http://kenicd.e-healthnet.gr/KEN%CE%91%CE%BD%CE%B1%CE%BB%CF%85%CF%84%CE%B9%CE%BA%CE%AC.aspx?iditem=%ce%a322%ce%9c. Accessed 30 June 2020.
Greek Ministry of Health. DRG code: Φ61Α. Diagnostic related groups. Available at: http://kenicd.e-healthnet.gr/KEN%CE%91%CE%BD%CE%B1%CE%BB%CF%85%CF%84%CE%B9%CE%BA%CE%AC.aspx?iditem=%ce%a661%ce%91. Accessed 30 June 2020.
Greek Ministry of Health. DRG code: Ρ22Μ. Diagnostic related groups. Available at: http://kenicd.e-healthnet.gr/KEN%CE%91%CE%BD%CE%B1%CE%BB%CF%85%CF%84%CE%B9%CE%BA%CE%AC.aspx?iditem=%ce%a122%ce%9c. Accessed 30 June 2020.
Gourzoulidis G, Vassilopoulos G, Gialama F, et al. Economic evaluation of dasatinib compared to nilotinib as second line treatment of chronic myeloid leukemia in Greece. Value Health. 2017;20(9):PA443.
National Organization for Healthcare Services Provision (EOPYY). Available at: https://www.eopyy.gov.gr/. Accessed 30 June 2020.
Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19:929–35. https://doi.org/10.1016/j.jval.2016.02.017.
Thokala P, Ochalek J, Leech AA, et al. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. 2018;36:509–22. https://doi.org/10.1007/s40273-017-0606-1.
Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828. https://doi.org/10.1080/16549716.2018.1447828.
Tzanetakos C, Stefanou G, Gourzoulidis G. PNS61 does a standard willingness-to-pay threshold exist in Greece? Value Health. 2019;22(Suppl 3):S772–3. https://doi.org/10.1016/j.jval.2019.09.1963.
International Monetary Fund. World Economic Outlook Database 2020. Available at: https://www.imf.org/external/pubs/ft/weo/2019/02/weodata/index.aspx. Accessed 30 June 2020
Tong WH, van der Sluis IM, Alleman CJ, et al. Cost-analysis of treatment of childhood acute lymphoblastic leukemia with asparaginase preparations: the impact of expensive chemotherapy. Haematologica. 2013;98(5):753–9. https://doi.org/10.3324/haematol.2012.073510.
Kurre HA, Ettinger AG, Veenstra DL, et al. A pharmacoeconomic analysis of pegaspargase versus native Escherichia coli l-asparaginase for the treatment of children with standard-risk, acute lymphoblastic leukemia: the Children’s Cancer Group study (CCG-1962). J Pediatr Hematol Oncol. 2002;24(3):175–81. https://doi.org/10.1097/00043426-200203000-00004.
Peters BG, Goeckner BJ, Ponzillo JJ, et al. Pegaspargase versus asparaginase in adult ALL: a pharmacoeconomic assessment. Formulary. 1995;30(7):388–93.
Acknowledgements
The authors would like to thank Servier Hellas for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Econcare LP received research grant funding from Servier Hellas. AB and VC were Servier Hellas employees at the time of this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed.
Funding
This study was sponsored by Servier Hellas. The study sponsor had no interference in the study design, data collection, or writing of the manuscript.
Ethical approval
This study was an economic evaluation analysis and does not involve human subjects. Input data including human material or human data derived from other published studies was performed with the approval of an appropriate Ethics Committee; therefore, ethics approval was not required for the performance of this cost-effectiveness analysis.
Consent to publish
Not applicable.
Consent to participate
Not applicable.
Code availability
Not applicable.
Availability of data and materials
All data used to conduct this study are included in this published article.
Author contributions
GG and MK conducted the analyses, collected the data, and interpreted the results. GG wrote the manuscript. AK, MB, and CG were the local clinical experts who provided local resource utilization data and contributed to data validation and interpretation of the results. VC, AB, and GK contributed to interpretation of the results and manuscript writing. All authors reviewed and approved the final version of the manuscript.
Additional information
The original online version of this article was revised: All authors’ first and last names have been swapped.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gourzoulidis, G., Koulentaki, M., Kattamis, A. et al. Cost-Utility Analysis of Pegaspargase for the Treatment of Acute Lymphoblastic Leukemia in Greece. Clin Drug Investig 42, 999–1008 (2022). https://doi.org/10.1007/s40261-022-01207-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01207-w