Abstract
Background and Objective
Trastuzumab emtansine (T-DM1) is the standard second-line option for the treatment of patients with human epidermal growth factor receptor-2 (HER2)-positive breast cancer for its superior clinical efficacy in prolonging progression-free survival. The objective of this study was to evaluate the cost effectiveness of T-DM1 from the Chinese healthcare perspective. Capecitabine (Cap), capecitabine + lapatinib (Cap + Lap), capecitabine + trastuzumab (Cap + Tra), capecitabine + trastuzumab + pertuzumab (Cap + Tra + Per) were selected as comparators.
Methods
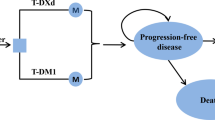
A three-state Markov simulation model was performed. The state transition probabilities were estimated based on the results of a published network meta-analysis, and utilities were derived from the published literature. The costs populated in the model were acquired from the local charge or previously published studies. One-way sensitive analysis and probabilistic sensitivity analyses were performed to test the robustness of the results.
Results
Compared with Cap, Cap + Lap, Cap + Tra, and Cap + Tra + Per, T-DM1 was estimated to increase the cost by US$109,699.1, $106,019.1, $97,506.3, and $67,121.9, respectively, and yield a gain of 0.544 quality-adjusted life years (QALYs), 0.383 QALYs, 0.367 QALYs, 0.087 QALYs, respectively. Corresponding incremental cost-effectiveness ratios (ICERs) were $201,652.9, $276,812.5, $265,685.0, and $771,516.1 per QALY. The probabilities of T-DM1 as the dominant option were 0% at the willingness-to-pay (WTP) threshold of $31,245.1/QALY.
Conclusions
T-DM1, as second-line therapy in the treatment of HER2-positive breast cancer, is not a cost-effective option in China. Given the significant clinical efficacy, an appropriate price reduction of T-DM1 is required to benefit more HER2-positive breast cancer patients.



Similar content being viewed by others
References
Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. Report of cancer epidemiology in China, 2015. Zhonghua zhong liu za zhi [Chin J Oncol]. 2019;41(1):19–28.
Wang L, Zhang Y, Shi JF, Dai M. Disease burden of female breast cancer in China. Zhonghua liu xing bing xue za zhi Zhonghua liuxingbingxue zazhi. 2016;37(7):970–6.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Yaziji H, Goldstein LC, Barry TS, Werling R, Hwang H, Ellis GK, Gralow JR, Livingston RB, Gown AM. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004;291(16):1972–7.
Wang BY, Ge R, Jiang ZF. Breast Cancer Expert Group of Chinese Society of Clinical O: [Expert consensus on the management of adverse events of ErbB family tyrosine kinase inhibitors in breast cancer]. Zhonghua zhong liu za zhi [Chin J Oncol]. 2020;42(10):798–806.
Escriva-de-Romani S, Arumi M, Bellet M, Saura C. HER2-positive breast cancer: current and new therapeutic strategies. Breast. 2018;39:80–8.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Krop IE, Kim SB, Martin AG, LoRusso PM, Ferrero JM, Badovinac-Crnjevic T, Hoersch S, Smitt M, Wildiers H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–54.
Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, Martin M, Pienkowski T, Pivot X, Burris H 3rd, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol. 2017;35(2):141–8.
Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, Krop IE, Blackwell K, Hoersch S, Xu J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–42.
Paracha N, Reyes A, Dieras V, Krop I, Pivot X, Urruticoechea A. Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis. Breast Cancer Res Treat. 2020;180(3):597–609.
Li R, Zhang L, Yang J, Cai Y, Chen W, Lan L, Xue M, Meng Q. Analysis of inpatient payments of breast cancer patients with different medical insurance coverages in China (mainland) in 2011–2015. Chin J Cancer Res Chung-kuo yen cheng yen chiu. 2017;29(5):419–25.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, Version 1. 2021. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 12 Mar 2021.
Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–43.
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006.
Urruticoechea A, Rizwanullah M, Im SA, Ruiz ACS, Lang I, Tomasello G, Douthwaite H, Badovinac Crnjevic T, Heeson S, Eng-Wong J, et al. Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J Clin Oncol. 2017;35(26):3030–8.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049.
Liu GN, Hu SL, Wu JH. China guidelines for pharmacoeconomic evaluations. China J Pharm Econ. 2011;3:6-9 + 11-48.
Engauge Digitizer. http://markummitchell.github.io/engauge-digitizer/. Accessed 16 Aug 2020.
The R Project for Statistical Computing. https://www.r-project.org/. Accessed 16 Aug 2020.
Lu S, Ye M, Ding L, Tan F, Fu J, Wu B. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget. 2017;8(6):9996–10006.
Andrew B, Karl C, Mark S. Decision modelling for health economic evaluation. New York: Oxford University Press Inc; 2006.
Ishak KJ, Kreif N, Benedict A, Muszbek N. Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics. 2013;31(8):663–75.
National Health Commission of China. Report on Nutrition and Chronic Disease Status of Chinese Residents(2015). http://www.nhc.gov.cn/jkj/s5879/201506/4505528e65f3460fb88685081ff158a2.shtml. Accessed 5 Aug 2020.
The Introduction of the Kadcyla charity program. http://www.cfchina.org.cn/show.php?contentid=2108. Accessed 16 Aug 2020.
Wang H, Zeng C, Li X, Wang Y, Li X, Ge W. Cost-utility of afatinib and gefitinib as first-line treatment for EGFR-mutated advanced non-small-cell lung cancer. Future Oncol. 2019;15(2):181–91.
Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. 2018;6(1):124.
Dranitsaris G, Yu B, King J, Kaura S, Zhang A. Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. ClinicoEcon Outcomes Res CEOR. 2015;7:249–56.
Diaby V, Adunlin G, Zeichner SB, Avancha K, Lopes G, Gluck S, Montero AJ. Cost-effectiveness analysis of everolimus plus exemestane versus exemestane alone for treatment of hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat. 2014;147(2):433–41.
Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer. 2018;17(4):e751–61.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
Le QA, Bae YH, Kang JH. Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2): positive advanced breast cancer. Breast Cancer Res Treat. 2016;159(3):565–73.
Yan H, Yu K, Zhang K, Liu L, Li Y. Efficacy and safety of trastuzumab emtansine (T-DM1) in the treatment of HER2-positive metastatic breast cancer (MBC): a meta-analysis of randomized controlled trial. Oncotarget. 2017;8(60):102458–67.
Elsada A, Doss S, Robertson J, Adam EJ. NICE guidance on trastuzumab emtansine for HER2-positive advanced breast cancer. Lancet Oncol. 2016;17(2):143–4.
Liao XZ, Shi JF, Liu JS, Huang HY, Guo LW, Zhu XY, Xiao HF, Wang L, Bai YN, Liu GX, et al. Medical and non-medical expenditure for breast cancer diagnosis and treatment in China: a multicenter cross-sectional study. Asia Pac J Clin Oncol. 2018;14(3):167–78.
Diaby V, Ali AA, Williams KJ, Ezendu K, Soto-Perez-de-Celis E, Chavarri-Guerra Y, de Lima LG. Economic evaluation of sequencing strategies in HER2-positive metastatic breast cancer in Mexico: a contrast between public and private payer perspectives. Breast Cancer Res Treat. 2017;166(3):951–63.
Wang LC, Kuo CN, Ko Y. Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in treating HER-2 positive advanced breast cancer in Taiwan. Breast J. 2020;26(10):2099–102
Giuliani J, Bonetti A. The cost-effectiveness of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer is supported by clinical evidence. Breast J. 2021;27(1):75–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This analysis received no funding.
Conflicts of interest
The authors declare that they have no potential conflicts of interest.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethics approval
This study was performed by a mathematical model, without any human participants or animals.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, Y., Huang, C. et al. Cost-effectiveness Analysis of Trastuzumab Emtansine as Second-line Therapy for HER2-Positive Breast Cancer in China. Clin Drug Investig 41, 569–577 (2021). https://doi.org/10.1007/s40261-021-01035-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01035-4