Abstract
Background and objective
The increasing availability of real-world evidence (RWE) about safety and effectiveness of direct non-vitamin K oral anticoagulants (DOACs) for the management of atrial fibrillation (AF) offers the opportunity to better understand the clinical and economic implications of DOACs versus vitamin K antagonists (VKAs). The objective of this study was to compare the economic implications of DOACs and VKAs using data from real-world evidence in patients with AF.
Methods
A Markov model simulating the lifetime course of patients diagnosed with non-valvular AF was used to evaluate the cost-effectiveness of DOACs (i.e., rivaroxaban, dabigatran and apixaban) versus VKAs from the Italian National Health System (INHS) perspective. The model was made up of data from the literature and a meta-analysis of RWE on the incidence of stroke/systemic embolism (SE), major bleeding (MB), intracranial haemorrhage (ICH) and all-cause mortality (ACM); direct costs included drug costs, costs for drug monitoring, and management of events from official national lists. One-way and probabilistic sensitivity analyses (PSA) were used to assess the robustness of the results.
Results
Results from the meta-analysis showed that apixaban had a high probability of being the most effective for stroke/SE, MB and ACM. Despite their higher acquisition costs, the cost-effectiveness analysis showed all DOACs involved a saving when compared with VKAs, with per-patient savings ranging between €4647 (rivaroxaban) to €6086 (apixaban). Moreover, all DOACs indicated a gain both in quality-adjusted life-years and life-years. According to PSA, findings related to apixaban were consistent, while for dabigatran and rivaroxaban PSA revealed a higher degree of uncertainty.
Conclusions
The beneficial effect of DOACs on containing events showed in RWE had the potential to offset drug-related costs, thus improving the sustainability of treatment for non-valvular AF in daily clinical practice.
Similar content being viewed by others
Sufficient evidence is now available to inform a cost-effectiveness analysis of direct non-vitamin K oral anticoagulants (DOACs) for the management of atrial fibrillation on the basis of real-word evidence. |
Synthetizing available real-world evidence studies, apixaban, dabigatran and rivaroxaban were likely to improve health benefit over warfarin. |
Despite the higher acquisition costs, apixaban was cost-effective compared to warfarin, suggesting savings for the Italian National Health System; considerable uncertainty still remained on the cost-effectiveness of dabigatran and rivaroxaban. |
1 Introduction
Atrial fibrillation (AF) is the most common and clinically significant arrhythmia, and one of the major causes of stroke, heart failure, sudden death and cardiovascular morbidity in the world; it also carries a significant cost burden as a result of treatment and frequent hospitalization as well as considerable impairment in quality of life (QoL) [1, 2].
According to published data, about 33 million people suffer from AF [3]. A recent study also highlights wide variability in the prevalence of AF worldwide with significant gender differences. Specifically, estimates for 2010 suggested an overall (age-adjusted) prevalence of AF of about 6.0 (95% confidence interval (CI) 5.6–6.4) per 1000 among men and 3.7 (95% CI 3.5–4.0) per 1000 among women; these figures were slightly higher in industrialized countries (compared to developing countries) being, respectively, 6.6 (95% CI 6.0–7.4) per 1000 among men and 3.9 (95% CI 3.4–4.5) per 1000 among women. Moreover, the USA and Canada showed the highest prevalence, while central and northern Europe, as well as India, Japan and China had the lowest rates, with data from eastern countries likely to be underestimated [1, 3].
Several studies suggested an increasing trend in the prevalence and incidence of AF in the last decades; that course could partially be explained by the aging population and the increasing prevalence of co-morbidities and cardiovascular risk factors, in addition to other factors such as lifestyle changes and improved diagnosis [1, 3].
Management of patients with AF requires an integrated approach to monitor and control the disease, but also to prevent disease burden.
Stroke prevention is crucial in AF patients, and the use of oral anticoagulants has been demonstrated to reduce the risk of events and also to decrease mortality [4,5,6]; indeed, the 2016 European Society of Cardiology (ESC) guidelines for AF recommend the use of oral anticoagulants in all patients with CHA2DS2-VASC risk factors ≥ 2 [7].
At present vitamin K antagonists (VKAs; i.e., warfarin) and four diverse non-vitamin K oral anticoagulants (DOACs)—apixaban, dabigatran, rivaroxaban and edoxaban—are available on the market, and can be used for stroke prevention in non-valvular AF (NVAF).
While treatment with VKAs represented the standard for effective stroke prevention for many years, their use requires constant monitoring of the anticoagulation effect through measurement of the International Normalized Ratio (INR) to ensure an optimal level; this results in physical, psychological, social and financial consequences for the patient and the healthcare team [7, 8].
On the other hand, DOACs eliminate the need for laboratory monitoring, and have minor drug and food interactions and a wide therapeutic window [9]. As a drawback, without the need for monitoring, adherence to DOACs cannot be easily assessed.
Because of their ease of use in routine clinical practice and their excellent efficacy and safety profile [10], since their first introduction, DOACs have rapidly became the mainstay of therapy for stroke prevention in patients with NVAF [10].
Specifically, results from randomized controlled trials showed a significant reduction in the risk of mortality, bleeding and stroke for apixaban versus VKAs, while results for the other DAOCs are not as clear for both safety and efficacy endpoints [11,12,13,14]. However, regarding their relative safety and effectiveness in routine care, results have not been homogeneous among different studies and a branch of evidence has suggested increased risk for gastrointestinal bleeding related to the use of DOACs [15, 16].
Despite the number of publications and clinical trials conducted to evaluate the efficacy and safety of DOACs, not much effort has been applied to investigating the clinical and economic impact of the use of these drugs using real-world data and even trying to compare the different DOACs, particularly in Italy.
Indeed, evidence from “real-world” experiences may not perfectly match results from controlled settings, leading to discrepancies in both effectiveness and costs associated with the use of different therapies [17,18,19].
Accordingly, the primary objective of this study was to estimate the long-term cost-effectiveness of stroke prevention in patients with NVAF comparing available DOACs with the standard treatment, VKAs, taking into consideration the perspective of the Italian National Health System (INHS).
To this end, specific objectives of the present study were the collection and synthesis of available real-world evidence comparing DOACs versus VKAs for the prevention of stroke and management of NVAF patients; on the basis of that data an economic evaluation was performed adapting to the Italian context a previously developed model [20]. This study provides an evaluation of the cost-effectiveness of available direct oral anticoagulation therapies to assist clinicians and other healthcare decision-makers to make a more informed choice regarding patients’ treatment.
2 Methods
A systematic literature review followed by a network meta-analysis was performed to synthesize the available evidence related to the effectiveness and safety of the use of DOACs for the management of NVAF patients; results from the meta-analysis were then used as effectiveness inputs for the cost-effectiveness analysis.
2.1 Systematic Literature Review and Network Meta-Analysis
2.1.1 Systematic Review
The systematic literature review aimed at identifying available evidence about the effectiveness and safety of DOACs versus VKAs for the management of NVAF patients in a real-world setting and was performed according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement method [21].
PubMed and Scopus databases were searched to retrieve studies published from 1 January 2009 to 31 September 2019 using the search strategies detailed in Online Supplementary Material (OSM) 1.
Studies published in scientific journals for which a full text was available in English and satisfying the inclusion and exclusion criteria reported in Table 1 were considered for inclusion in the systematic review.
In brief, retrieved studies were included if they referred to real-world studies comparing DOACs versus VKAs for the management of NVAF patients with CHA2DS2-VASC risk factors ≥ 2 and reported adjusted results (i.e., comparing propensity score-matched groups, multivariate models) related to the effect of the diverse treatments used at standard doses (apixaban 5 mg twice a day, dabigatran 150 mg twice a day, and rivaroxaban 20 mg once a day) on at least one of the following endpoints: stroke/systemic embolism (SE), major bleeding (MB), intracranial haemorrhage and all-cause mortality (ACM). Studies considering mixed populations treated with doses that were different from the standard were included if specific data for the different dosages were available, more than 50% of the population was treated at the standard dose, or if there was no clear evidence that the majority of the study population received doses different from the standard ones. Randomized trials or other experimental studies, studies considering specific subgroups of patients (i.e., NVAF patients with associated kidney disease, NVAF with CHA2DS2-VASC risk factors < 2), or those for which a standard and clear definition of events (i.e., referring to the specific ICD-9 or ICD-10 codes) was not available were excluded.
Two authors (SP and VL) independently performed the literature search, and screened retrieved studies for eligibility on the basis of titles (first step), abstracts (second step) and full text (third step). Any discrepancies were resolved involving a third author (GT) and reaching consensus.
The following data were obtained from studies included in the analysis: study characteristics, patients’ characteristics and outcomes; the number of events and population size or adjusted hazard ratio (HR) and its 95% CI were extracted and used in the network meta-analysis.
2.1.2 Network Meta-Analysis
A network meta-analysis was thus performed to combine and synthetize evidence from the systematic literature review.
Network meta-analysis allows combining data from different studies assuming that within-studies estimates of relative treatment effects could be obtained as differences in the between-treatment effects, with the appropriate choice of a measurement scale.
The meta-analysis was performed considering the approach proposed by Woods et al. [22], which allows combining HR and cumulative count survival data on the log hazard scale and considering a Bayesian framework. The approach used also accounted for the correlations in relative treatment effect estimates that arise from trials with more than two treatment arms—multi-arm trials. Separated models were adapted for each endpoint of interest and analyses were performed using WinBUGS 1.4.3.
Each model was run for 50,000 burn-in simulations followed by an additional 200,000 runs. Two sets of initial values were used in all models and convergence was evaluated by trace plot; autocorrelation was also assessed. In addition, both fixed and random-effects models were considered for each endpoint of interest and the deviance information criteria (DIC) was used to compare the model fit. On the basis of the DIC value and on the estimates of the random-effect parameter, results from fixed models were retained for the analysis as there was no evidence concerning the significance of the random effect.
Results from the meta-analysis are expressed as HR and 95% credible interval (CrI); for each endpoint, ranking of the diverse alternatives was also derived from the model to provide a rank of the diverse treatment approaches.
2.2 Cost-Effectiveness Analysis
The cost-effectiveness analysis was performed on the basis of a Markov model considering a lifetime horizon and the INHS perspective. Results were reported using the incremental cost-utility ratio (ICUR) expressed as cost per quality-adjusted life-years (QALY) and the incremental cost-effectiveness ratio (ICER) expressed as cost per life-year gained (LYG). All costs and outcomes were discounted at 3.5% per year.
2.2.1 Model Structure and Perspectives
The economic evaluation was performed adapting a model previously developed as a Microsoft Excel enabled workbook; details about the structure of the model have been previously reported [20].
Adaptation of the previously developed model was performed to adjust it to the context of the INHS (a system providing universal coverage to citizens and residents, with public healthcare largely free of charge) and to account for the incidence of events from real-world studies.
The model evaluates and compares the use of DOACs for the prevention of stroke in AF and allows comparisons of each DOAC versus a VKA but also compares the cost-effectiveness of the diverse DOACs.
Markov transitions are employed to describe the disease progression, with a 3-monthly cycle.
A schematic representation of the model structure is provided in Fig. 1.
In brief the model mimics the disease course of a hypothetical cohort of 1000 patients diagnosed with NVAF. Patients entering the model because of the diagnosis of NVAF had predefined risks of events according to their actual condition [20, 23] that were modified according to the treatment arm they entered in. Accordingly, from the initial state “AF well” patients move to different states characterized by the number of events they experience—“AF + single event”, “AF + two events”, “AF + three events” or even death. Stroke/SE, major bleed (MB) or intracranial haemorrhage (ICH) were considered as events, and transition probabilities were defined on the basis of the number and type of events experienced.
Four different treatment arms were considered in the analysis, representing first-line treatment with warfarin, apixaban, dabigatran and rivaroxaban. Edoxaban was not considered in the present analysis as just one study was retrieved from the literature search performed.
The following assumptions were also considered in the model: (1) patients always switch to no treatment after haemorrhage; (2) patients may switch from DOACs to warfarin or from warfarin to no treatment after stroke, major bleed or systemic embolism; (3) patients may switch from DOACs to warfarin or discontinue warfarin at any time due to lack of compliance.
The model estimates annual direct health costs for the different treatment options considering costs associated with oral anticoagulant therapy, costs related to hospitalizations for the management of events and monitoring costs, where appropriate.
2.2.2 Effectiveness and Cost Data
Inputs for effectiveness were derived from the meta-analysis on real-word experience with the use of DOACs versus VKAs described above. On the basis of mortality and the risk of events, life-years (LYs) and QALYs for the diverse treatments were thus obtained from the model. To derive QALYs, utilities were applied according to previously published data referring to the events of interests and considering a utility value for stable AF equal to 0.779 [24,25,26]; details of the values used in the analysis are reported in Table 2.
Direct health costs related to the oral anticoagulant therapy (either VKAs and DOACs), direct health costs of events and eventual monitoring costs were considered in the analysis.
Specifically, annual cost associated with the oral anticoagulant therapy was estimated multiplying, for the different treatment options, daily standard dosage times unit costs of the product considering ex-factory prices from the National Agency for Drugs [27] and from the National Gazette [28,29,30], including statutory discount. For VKAs, costs of monitoring were also added to the cost of drugs to fully capture all treatment costs; costs of INR monitoring in the Italian setting were derived from previously published data and inflated to the reference year [31, 32].
Costs associated with the incidence of events were estimated considering annual incidence rate and reimbursement associated with hospitalization for the management of each event according to official national charges set by the Italian Ministry of Health (i.e., Tariffario prestazioni assistenza ospedaliera per acuti) [33]. Only for stroke costs associated with the event also included costs of rehabilitation derived from a previous study conducted in Italy [34] and inflated to the reference year. Details of cost inputs used are reported in Table 3.
All costs are expressed in Euros and referred to 2019 values.
2.2.3 Sensitivity Analysis
To evaluate the robustness of results both one-way (OWSA) and probabilistic sensitivity analyses were conducted. One-way sensitivity analysis was conducted on all model parameters associated with uncertainty, modifying base-case inputs by ± 20% and showing main results from the analysis in a Tornado diagram. In the probabilistic sensitivity analysis (PSA), costs, utilities and transition probabilities were varied simultaneously over their 95% CIs (see OSM 2) and ICURs and ICERs were calculated over 1000 simulations. Results from the PSA were presented in a cost-effectiveness plane.
3 Results
3.1 Systematic Literature Review and Network Meta-Analysis
Articles identified through the literature searches were checked to identify any duplicates. Then reviewers screened titles and abstracts and selected publications that met the eligibility criteria; fulltexts of selected references were then assessed.
A total of 581 references were identified through the searching strategy, 494 were identified in Pubmed, 84 from Scopus and three additional studies were added as being previously known.
After excluding duplicates, a total of 568 references were independently screened and according to pre-defined inclusion and exclusion criteria 424 studies were excluded on the basis of title or abstract. Finally, 144 full texts were evaluated, and among these overall 42 studies were included in the meta-analysis. Figure 2 provides the flow diagram followed in our review to select the retrieved studies.
Data for each outcome were extracted and organized by the standard dosages and evaluated in the meta-analysis.
Details of studies included the meta-analysis and a summary of main details in terms of study type, interventions considered and the type of endpoints analysed are reported in OSM 3.
Accordingly 26 studies were considered for stroke/SE [16, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], 29 for MB [16, 36, 40,41,42,43,44, 46,47,48, 50, 52, 54,55,56, 58, 61,62,63,64,65,66,67,68,69,70,71,72], 20 for ICH [36, 38, 41,42,43, 45, 49,50,51,52, 54, 55, 60, 63, 64, 71,72,73,74,75,76] and 13 for ACM [16, 36, 41,42,43, 46, 52,53,54, 67, 71, 72, 77, 78].
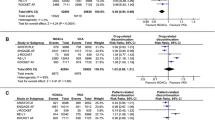
Results from the meta-analysis suggested that for each endpoint all DOACs significantly reduced the risk of events and mortality; only when considering major bleeding the network meta-analysis suggested that rivaroxaban had a higher probability of implying a slight increased risk as compared to VKAs (Table 4).
Trying to rank the effectiveness and safety profile of the different treatments with respect to events considered in the analysis, apixaban resulted in having a high probability of being the best option for both stroke/SE, MB and ACM. On the other hand, for ICH dabigatran showed a higher probability of being the best alternative (Table 5).
3.2 Cost-Effectiveness Analysis
Despite the lower acquisition costs of the drug, overall life-time direct costs estimated through the model were higher for warfarin, €21,331 per patient, while apixaban resulted in the lowest one, €15,245 per patient. Costs associated with dabigatran and rivaroxaban were slightly higher than those associated with apixaban, €16,003 and €16,684 per patient, respectively (Table 6).
As shown in Fig. 3, the overall costs associated with warfarin were mainly driven by management costs that also comprised costs for INR monitoring; the rest were almost all event costs. On the contrary, costs associated with DOACs were for the large part equally driven by events costs and drug costs.
The cost-effectiveness analysis showed that all DOACs lead to savings when compared with VKAs, resulting in a reduction of per patient cost ranging between €4647 (rivaroxaban) and €6086 (apixaban). Dabigatran incurred a reduction in per patient costs of about €5327 when compared with VKAs. Moreover, all DOACs implied a gain in both QALYs and LYs.
Accordingly, when compared to VKAs all DOACs resulted in dominantly negative values for ICURs and ICERs, induced by lower costs and higher effectiveness (Table 6).
On the other hand, comparisons among the different DOACs highlighted that apixaban had the lowest per-patient costs, with QALYs and LYs that were slightly higher than those seen with the other DOACs (Table 7).
As compared to apixaban, rivaroxaban and dabigatran resulted in higher costs and lower effectiveness (in term of both LYs and QALYs).
The main results from the OWSA are shown in Fig. 4. When comparing DOACs and VKAs, for all DOACs drug costs were among the main drivers of the analysis; variation in the HR for ACM also had a sensible impact on results from the base-case analysis, particularly when comparing apixaban and dabigatran versus VKAs. Results from the PSA suggested that results obtained from the base-case analysis showed a high degree of uncertainty with regard to the results from the cost-effectiveness analysis related to the comparison of dabigatran and rivaroxaban versus VKAs, while the scatter cloud related to apixaban was almost consistently located in the second quadrant, suggesting robustness of the findings from the base-case analysis (Fig. 5).
4 Discussion
To our knowledge this is the first study conducted to assess and compare the cost-effectiveness of the use of different DOACs versus VKAs considering a real-world setting and the Italian context.
Excluding edoxaban, which has been launched more recently, and for which just a few studies were available at the time of the analysis, with just one meeting the inclusion and exclusion criteria, at present there is sufficient real-world evidence to support the use of other DOACs in terms of both effectiveness and costs.
Results from the present study highlight a clear benefit related to the use of DOACs versus VKAs because of the higher QALYs and LYs induced by their effectiveness and safety profile and also their lower costs; indeed, the higher acquisition costs of DOACs were offset by reduced costs associated with management of events and patient monitoring. Specifically, on the basis of the analyses performed, apixaban resulted in higher benefits as compared to the other DOACs.
Results from the cost-effectiveness analysis also suggest the potential cost-effectiveness of all DOACs, although PSA clearly indicated that only for apixaban results were consistent, while a high degree of uncertainty was noted for both dabigatran and rivaroxaban.
Few other studies have been conducted focusing on the cost-effectiveness of DAOCs in a real-world setting [18, 79], while a consistent branch of evidence deals with the cost-effectiveness of these drugs considering data from RCTs [80,81,82,83,84].
Informing decision-making evidence from real-world settings is essential, even if not required from regulatory bodies, to adjust the relative efficacy results from RCTs to the actual performance of the comparator, to better contextualize the analysis to specific settings (i.e., in terms of patient characteristics, treatment scenarios), and to expand findings beyond the trial duration [18].
Findings from the present study are somewhat in line with a recent cost-effectiveness analysis on real-world evidence aimed at evaluating the use of available DOACs (vs. VKAs) considering the perspective of the Dutch National Health System [79].
As real-world practice may largely vary across different contexts, a limitation of the present study is that the analysis performed is based on real-world data from diverse contexts. Despite inclusion and exclusion criteria used for the identification of evidence included in the meta-analysis were defined also to ensure the inclusion of studies being as similar as possible (in terms of study design, patients, etc.), being real-world studies reporting evidence from clinical practice in different contexts a certain degree of homogeneity in the evidence cannot be excluded. Moreover, not enough data are available at present to perform a comprehensive cost-effectiveness analysis based on robust evidence in the Italian context. Future studies, when feasible, could offer more specific insight into the Italian context.
Another study limitation lies in the fact that the model did not specifically account for adherence with the different DOACs as limited and inconsistent data were found from the systematic literature review. Indeed, despite it being unfeasible to model the impact of adherence on drug-related costs and on other direct health costs, being based on real-world studies, we believe the evidence used in the meta-analysis performed allow us to take into account the possible effect of suboptimal adherence behaviour on the incidence of events.
This point merits further study as adherence in a real-world setting could strongly impact the effectiveness and safety of drugs, and thus also on events rate and costs. Moreover, as only few real-world studies reported data on the impact of the diverse treatments on both myocardial infarction (MI) and transient ischaemic attack (TIA), these events were not considered in the model. Despite this resulting in a partial evaluation of all possible costs associated with the different treatments, that is true for all the options considered in the present study, thus not favouring any specific option; moreover, data retrieved from the systematic literature review, mainly related to dabigatran versus VKAs, suggested the incidence of both MI and TIA to be generally not different [16, 53, 54, 56, 57, 85, 86]. Another limitation of the study was the inability to include edoxaban to provide a comprehensive evaluation of the impact of all currently available approaches. Indeed, in a recent study reporting a meta-analysis and cost-effectiveness evaluation performed focusing on RCTs, edoxaban was shown to have a favourable effectiveness profile [23], resulting in being ranked as the second best after apixaban, in view of its effect related to the risk reduction for both MB and mortality versus VKA.
Despite the limitations presented, the present study adds to the limited evidence produced on the cost-effectiveness of DOACs in the real-world, contributing to the removal of uncertainties about DOACs [84] and also providing useful data for both clinicians and decision makers in Italy not only on the use of DOACs compared to VKAs, but also providing suggestions for choosing the most effective and sustainable options among actually available alternatives.
5 Conclusions
Based on up-to-date evidence from real-world studies, the present analysis suggests that apixaban is a cost-saving alternative to warfarin therapy for the prevention of stroke in patients with AF in the Italian healthcare setting; however, considerable uncertainty still remains on the cost-effectiveness of dabigatran and rivaroxaban.
Change history
15 April 2021
Former problem: Funding information was missing and Correct information: Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement.
References
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–47.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: the Task Force for the Management of Valvular Heart Disease. Eur Heart J. 2017;38(36):2739–86.
Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–54.
Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention of thromboemolic events-European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16(1):6–14.
Kirchhof P, Nabauer M, Gerth A, Limbourg T, Lewalter T, Goette A, et al. Impact of the type of centre on management of AF patients: surprising evidence for differences in antithrombotic therapy decisions. Thromb Haemost. 2011;105(6):1010–23.
Lip GYH, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J. 2014;35(47):3365–76.
Kirchhof P, Benussi S, Zamorano JL, Aboyans V, Achenbach S, Agewall S, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Russ J Cardiol. 2017;147:7–86.
Gadisseur APA, Kaptein AA, Breukink-Engbers WGM, Van Der Meer FJM, Rosendaal FR. Patient self-management of oral anticoagulant care vs. management by specialized anticoagulation clinics: positive effects on quality of life. J Thromb Haemost. 2004;2(4):584–91.
Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–77.
Husted S, de Caterina R, Andreotti F, Arnesen H, Bachmann F, Huber K, et al. Non-vitamin K antagonist oral anticoagulants (NOACs): No longer new or novel. Thromb Haemost. 2014;111:781–2.
Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Mayer F, Kirchmayer U, Coletta P, Agabiti N, Belleudi V, Cappai G, et al. Safety and effectiveness of direct oral anticoagulants versus vitamin K antagonists: pilot implementation of a near-real-time monitoring program in Italy. J Am Heart Assoc. 2018;7(6):e008034.
Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, et al. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290–8.
Freedman B, Lip GYH. “Unreal world” or “real world” data in oral anticoagulant treatment of atrial fibrillation. Thromb Haemost. 2016;116:587–9.
de Pouvourville G, Blin P, Karam P. The contribution of real-world evidence to cost-effectiveness analysis: case study of Dabigatran etexilate in France. Eur J Health Econ. 2020;21(2):235–49.
Makady A, van Veelen A, Jonsson P, Moseley O, D’Andon A, de Boer A, et al. Using real-world data in health technology assessment (HTA) practice: a comparative study of five HTA agencies. Pharmacoeconomics. 2018;36(3):359–68.
López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54.
Sterne JA, Bodalia PN, Bryden PA, Davies PA, Lopez-Lopez JA, Okoli GN, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2017;21:1–386.
Robinson A, Thomson R, Parkin D, Sudlow M, Eccles M. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy. 2001;6(2):92–8.
Lacey EA, Walters SJ. Continuing inequality: gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health. 2003;57(8):622–7.
Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Informatics Assoc. 1997;4(1):49–56.
Elenchi farmaci di classe A e H | Agenzia Italiana del Farmaco [Internet]. [cited 2020 Jun 14]. https://www.aifa.gov.it/liste-farmaci-a-h.
Gazzetta Ufficiale [Internet]. [cited 2020 Jun 14]. https://www.gazzettaufficiale.it/eli/id/2019/03/06/19A01576/sg.
Gazzetta Ufficiale [Internet]. [cited 2020 Jun 14]. https://www.gazzettaufficiale.it/eli/id/2019/03/06/19A01575/sg.
Gazzetta Ufficiale [Internet]. [cited 2020 Jun 14]. https://www.gazzettaufficiale.it/eli/id/2019/03/06/19A01565/sg.
Pradelli L, Calandriello M, Di Virgilio R, Bellone M, Tubaro M. The economic impact associated with cerebrovascular events related to non-valvular atrial fibrillation (NVAF) in Italy: the role of apixaban. Farmeconomia Heal Econ Ther pathways. 2014;15(1S):3–4.
Mennini F, Russo S, Marcellusi A. Budget impact analysis resulting from the use of dabigatran etexilate in preventing stroke in patients with non-valvular atrial fibrillation in Italy. Farmeconomia Heal Econ Ther Pathways. 2012;13(3):121–31.
Italian Ministry of Health. Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. Gazz Uff Ser Gen. 2013.
Piscitelli P, Iolascon G, Argentiero A, Chitano G, Neglia C, Marcucci G, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging. 2012;7:575–83.
Larsen TB, Rasmussen LH, Gorst-Rasmussen A, Skjoth F, Lane DA, Lip GYH. Dabigatran and warfarin for secondary prevention of stroke in atrial fibrillation patients: a nationwide cohort study. Am J Med. 2014;127:1172-1178.e5.
Forslund T, Wettermark B, Andersen M, Hjemdahl P. Stroke and bleeding with non-Vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non-valvular atrial fibrillation: a population-based cohort study. Europace. 2018;20(3):420–8.
Deitelzweig S, Luo X, Gupta K, Trocio J, Mardekian J, Curtice T, et al. Comparison of effectiveness and safety of treatment with apixaban vs. other oral anticoagulants among elderly nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2017;33(10):1745–54.
Hernandez I, Zhang Y, Brooks MM, Chin PKL, Saba S. Anticoagulation use and clinical outcomes after major bleeding on dabigatran or warfarin in atrial fibrillation. Stroke. 2017;48:159–66.
Staerk L, Fosbøl EL, Lip GYH, Lamberts M, Bonde AN, Torp-Pedersen C, et al. Ischaemic and haemorrhagic stroke associated with non-Vitamin K antagonist oral anticoagulants and warfarin use in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J [Internet]. Oxford University Press; 2017;38:907–15. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85015880382&doi=10.1093%2Feurheartj%2Fehw496&partnerID=40&md5=ef5d24caa30b858dfcf6c1fa0cfbcc0d.
Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725.
Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke. 2018;49(12):2933–44.
Correction to: effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke. 2020;51(4):e71.
Hohnloser SH, Basic E, Hohmann C, Nabauer M. Effectiveness and safety of non-Vitamin K oral anticoagulants in comparison to phenprocoumon: data from 61,000 patients with atrial fibrillation. Thromb Haemost. 2018;118(3):526–38.
Korenstra J, Wijtvliet EPJ, Veeger NJGM, Geluk CA, Bartels GL, Posma JL, et al. Effectiveness and safety of dabigatran versus acenocoumarol in “real-world” patients with atrial fibrillation. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2016;18:1319–27.
Norby FL, Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Chamberlain AM, et al. Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. 2017;17:238.
Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GYH. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:3189.
Gupta K, Trocio J, Keshishian A, Zhang Q, Dina O, Mardekian J, et al. Real-world comparative effectiveness, safety, and health care costs of oral anticoagulants in nonvalvular atrial fibrillation patients in the U.S. department of defense population. J Manag Care Spec Pharm. 2018;24(11):1116–27.
Amin A, Keshishian A, Trocio J, Dina O, Le H, Rosenblatt L, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33:1595–604.
Bengtson LGS, Lutsey PL, Chen LY, MacLehose RF, Alonso A. Comparative effectiveness of dabigatran and rivaroxaban versus warfarin for the treatment of non-valvular atrial fibrillation. J Cardiol. 2017;69(6):868–76.
Martinez BK, Baker WL, Sood NA, Bunz TJ, Meinecke A-K, Eriksson D, et al. Influence of polypharmacy on the effectiveness and safety of rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation. Pharmacotherapy. 2019;39(2):196–203.
Coleman CI, Antz M, Bowrin K, Evers T, Simard EP, Bonnemeier H, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;32:2047–53.
Själander S, Sjögren V, Renlund H, Norrving B, Själander A. Dabigatran, rivaroxaban and apixaban vs. high TTR warfarin in atrial fibrillation. Thromb Res. 2018;167:113–8.
Yavuz B, Ayturk M, Ozkan S, Ozturk M, Topaloglu C, Aksoy H, et al. A real world data of dabigatran etexilate: multicenter registry of oral anticoagulants in nonvalvular atrial fibrillation. J Thromb Thrombolysis. 2016;42:399–404.
Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–73.
Laliberte F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30:1317–25.
Bouillon K, Bertrand M, Maura G, Blotiere P-O, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2:e150–9.
Seeger JD, Bykov K, Bartels DB, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114:1277–89.
Maura G, Blotiere P-O, Bouillon K, Billionnet C, Ricordeau P, Alla F, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132:1252–60.
Coleman CI, Turpie AGG, Bunz TJ, Eriksson D, Sood NA, Baker WL. Effectiveness and safety of rivaroxaban versus warfarin in nonvalvular atrial fibrillation patients with a non-sex-related CHA2DS2-VASc score of 1. Eur Heart J Cardiovasc Pharmacother. 2019;5(2):64–9.
Li X, Keshishian A, Hamilton M, Horblyuk R, Gupta K, Luo X, et al. Apixaban 5 and 2.5 mg twice-daily versus warfarin for stroke prevention in nonvalvular atrial fibrillation patients: Comparative effectiveness and safety evaluated using a propensity-score-matched approach. PLoS ONE. 2018;13:e0191722.
Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
Yap LB, Eng DTS, Sivalingam L, Rusani BI, Umadevan D, Muhammad Z, et al. A comparison of dabigatran with warfarin for stroke prevention in atrial fibrillation in an Asian population. Clin Appl Thromb Hemost. 2016;22:792–7.
Coleman CI, Bunz TJ, Eriksson D, Meinecke A-K, Sood NA. Effectiveness and safety of rivaroxaban vs warfarin in people with non-valvular atrial fibrillation and diabetes: an administrative claims database analysis. Diabet Med. 2018;35:1105–10.
Li XS, Deitelzweig S, Keshishian A, Hamilton M, Horblyuk R, Gupta K, et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. A propensity-matched analysis of 76,940 patients. Thromb Haemost. 2017;117:1072–82.
Lip GYH, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin: a propensity score matched analysis. Thromb Haemost. 2016;116(5):975–86.
Adeboyeje G, Sylwestrzak G, Barron JJ, White J, Rosenberg A, Abarca J, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag care Spec Pharm. 2017;23:968–78.
Lee KH, Park HW, Lee N, Hyun DY, Won J, Oh SS, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2017;19:iv1–9.
Ramagopalan S, Allan V, Saragoni S, Esposti LD, Alessandrini D, Perrone V, et al. Patient characteristics and bleeding events in nonvalvular atrial fibrillation patients treated with apixaban or vitamin K antagonists: real-world evidence from Italian administrative databases. J Comp Eff Res. 2018;7:1063–71.
Russo-Alvarez G, Martinez KA, Valente M, Bena J, Hu B, Luxenburg J, et al. Thromboembolic and Major Bleeding Events With Rivaroxaban Versus Warfarin Use in a Real-World Setting. Ann Pharmacother United States. 2018;52:19–25.
Lamberts M, Staerk L, Olesen JB, Fosbøl EL, Hansen ML, Harboe L, et al. Major bleeding complications and persistence with oral anticoagulation in non-valvular atrial fibrillation: contemporary findings in real-life Danish patients. J Am Heart Assoc. 2017;6(2):e004517.
Graham DJ, Baro E, Zhang R, Liao J, Wernecke M, Reichman ME, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.
Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505.
Halvorsen S, Ghanima W, Tvete IF, Hoxmark C, Falck P, Solli O, et al. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur Heart J Cardiovasc Pharmacother. 2017;3(1):28–36.
S. D, A. K, X. L, M. H, C. M, K. G, et al. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban among non-valvular atrial fibrillation patients: A propensity score matched analysis of four large databases. Circulation [Internet]. S. Deitelzweig, Dept of Hosp Medicine, Ochsner Clinic Foundation, New Orleans, LA, United States; 2017;136. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L619986052.
Huang HY, Lin SY, Cheng SH, Wang CC. Effectiveness and safety of different rivaroxaban dosage regimens in patients with non-valvular atrial fibrillation: a nationwide, population-based cohort study. Sci Rep. 2018;8(1):3451.
Ellis MH, Neuman T, Bitterman H, Dotan SG, Hammerman A, Battat E, et al. Bleeding in patients with atrial fibrillation treated with dabigatran, rivaroxaban or warfarin: a retrospective population-based cohort study. Eur J Intern Med. 2016;33:55–9.
Shantha GPS, Bhave PD, Girotra S, Hodgson-Zingman D, Mazur A, Giudici M, et al. Sex-specific comparative effectiveness of oral anticoagulants in elderly patients with newly diagnosed atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2017;10(4):e003418.
Vaughan Sarrazin MS, Jones M, Mazur A, Chrischilles E, Cram P. Bleeding rates in veterans affairs patients with Atrial fibrillation who switch from Warfarin to Dabigatran. Am J Med. 2014;127(12):1179–85.
de Jong LA, Gout-Zwart JJ, van den Bosch M, Koops M, Postma MJ. Rivaroxaban for non-valvular atrial fibrillation and venous thromboembolism in the Netherlands: a real-world data based cost-effectiveness analysis. J Med Econ [Internet]. 2019. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85060129162&doi=10.1080%2F13696998.2018.1563404&partnerID=40&md5=b196f4b9c05487232216f07a89b5fd89.
Clemens A, Peng S, Brand S, Brueckmann M, Kansal A, Lim J, et al. Efficacy and cost-effectiveness of dabigatran etexilate versus warfarin in atrial fibrillation in different age subgroups. Am J Cardiol. 2014;114:849–55.
Salata BM, Hutton DW, Levine DA, Froehlich JB, Barnes GD. Cost-effectiveness of dabigatran (150 mg twice daily) and warfarin in patients >/= 65 years with nonvalvular atrial fibrillation. Am J Cardiol. 2016;117:54–60.
Li X, Tse VC, Lau WCY, Cheung BMY, Lip GYH, Wong ICK, et al. Cost-effectiveness of apixaban versus warfarin in Chinese patients with non-valvular atrial fibrillation: a real-life and modelling analyses. PLoS ONE. 2016;11:e0157129.
Kleintjens J, Li X, Simoens S, Thijs V, Goethals M, Rietzschel ER, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics. 2013;31(10):909–18.
Wisløff T, Hagen G, Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics. 2014;32(6):601–12.
Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–64.
Lee H-F, See L-C, Li P-R, Liu J-R, Chao T-F, Chang S-H, et al. Non-vitamin K antagonist oral anticoagulants and warfarin in atrial fibrillation patients with concomitant peripheral artery disease. Eur Heart J Cardiovasc Pharmacother. 2021;7(1):50–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement. The study received support from Pfizer Italia and Bristol Myers Squibb Italia.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The data used in this study are fully available in databases.
Code availability
Not applicable.
Conflict of interest
VL, SP and GT are employees of the Institute of Management, Scuola Superiore Sant’Anna, which received financial support from Pfizer and Bristol Myers Squibb in connection with the development of this article. The authors have no other conflicts of interest to declare.
Author contributions
VL conceived the paper and performed the analysis. SP performed the literature search, all the authors were involved in the systematic literature review. All authors were involved in the study design, discussion and interpretation of the results. All authors repeatedly edited the manuscript and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lorenzoni, V., Pirri, S. & Turchetti, G. Cost-Effectiveness of Direct Non-Vitamin K Oral Anticoagulants Versus Vitamin K Antagonists for the Management of Patients with Non-Valvular Atrial Fibrillation Based on Available “Real-World” Evidence: The Italian National Health System Perspective. Clin Drug Investig 41, 255–267 (2021). https://doi.org/10.1007/s40261-021-01002-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01002-z