Abstract
Background and Objective
Treatment challenges necessitate new approaches to customize care to individual patient needs. Integrating data from randomized controlled trials and observational studies may reduce potential covariate biases, yielding information to improve treatment outcomes. The objective of this study was to predict pregabalin responses, in individuals with painful diabetic peripheral neuropathy, by examining time series data (lagged inputs) collected after treatment initiation vs. baseline using microsimulation.
Methods
The platform simulated pregabalin-treated patients to estimate hypothetical future pain responses over 6 weeks based on six distinct time series regressions with lagged variables as inputs (hereafter termed “time series regressions”). Data were from three randomized controlled trials (N = 398) and an observational study (N = 3159). Regressions were derived after performing a hierarchical cluster analysis with a matched patient dataset from coarsened exact matching. Regressions were validated using unmatched (observational study vs. randomized controlled trial) patients. Predictive implications (of 6-week outcomes) were compared using only baseline vs. 1- to 2-week prior data.
Results
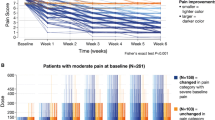
Time series regressions for pain performed well (adjusted R2 0.85–0.91; root mean square error 0.53–0.57); those with only baseline data performed less well (adjusted R2 0.13–0.44; root mean square error 1.11–1.40). Simulated patient distributions yielded positive predictive values for > 50% pain score improvements from baseline for the six clusters (287–777 patients each; range 0.87–0.98).
Conclusions
Effective prediction of pregabalin response for painful diabetic peripheral neuropathy was accomplished through combining cluster analyses, coarsened exact matching, and time series regressions, reflecting distinct patterns of baseline and “on-treatment” variables. These results advance the understanding of microsimulation to predict patient treatment responses through integration and inter-relationships of multiple, complex, and time-dependent characteristics.
Plain Language Summary
Analyzing the tremendous amount of patient data can provide meaningful insights to improve healthcare quality. Using statistical methods to combine data from clinical trials with real-world studies can improve overall data quality (e.g., reducing biases related to real-world patient variability).
AbstractSection Why Consider a Time Series Analysis?The best predictor of future outcomes is past outcomes. A “time series” collects data at regular intervals over time. Statistical analyses of time series data allow us to discern time-dependent patterns to predict future clinical outcomes. Modeling and simulation make it possible to combine enormous amounts of data from clinical trial databases to predict a patient’s clinical response based on data from similar patients. This approach improves selecting the right drug/dose for the right patient at the right time (i.e., personalized medicine). Using modeling and simulation, we predicted which patients would show a positive response to pregabalin (a neuropathic pain drug) for painful diabetic peripheral neuropathy.
AbstractSection What are the Major Findings and Implications?For pregabalin-treated patients, a time series analysis had substantially more predictive value vs. analysis only of baseline data (i.e., data collected at treatment initiation). The ability to best predict which patients will respond to therapy has the overall implication of better informing drug treatment decisions. For example, an appropriate modeling and simulation platform complete with relevant historical clinical data could be integrated into a stand-alone device used to monitor and also predict a patient’s response to therapy based on daily outcome measures (e.g., smartphone apps, wearable technologies).
Similar content being viewed by others
References
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73.
Juhn MS, Parsons B, Varvara R, Sadosky A. Pregabalin for painful diabetic peripheral neuropathy: strategies for dosing, monotherapy vs. combination therapy, treatment-refractory patients, and adverse events. Curr Med Res Opin. 2015;31:1017–26.
Borsook D, Kalso E. Transforming pain medicine: adapting to science and society. Eur J Pain. 2013;17:1109–25.
Gereau RW 4th, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, et al. A pain research agenda for the 21st century. J Pain. 2014;15:1203–14.
Stanos S, Brodsky M, Argoff C, Clauw DJ, D’Arcy Y, Donevan S, et al. Rethinking chronic pain in a primary care setting. Postgrad Med. 2016;128:502–15.
Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19–25.
Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind. COMBO-DN study. Pain. 2014;155:2171–9.
Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–76.
Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005.
Markman JD, Jensen TS, Semel D, Li C, Parsons B, Behar R, et al. Effects of pregabalin in patients with neuropathic pain previously treated with gabapentin: a pooled analysis of parallel-group, randomized, placebo-controlled clinical trials. Pain Pract. 2017;17:718–28.
Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92.
Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–86.
David Eddy created the Archimedes model to predict and analyze care. Health Aff (Millwood). 2012;31:2451–2. https://doi.org/10.1377/hlthaff.2012.1063 (PubMed PMID: 23129675).
Birnbaum JK, Ademuyiwa FO, Carlson JJ, Mallinger L, Mason MW, Etzioni R. Comparative effectiveness of biomarkers to target cancer treatment: modeling implications for survival and costs. Med Decis Making. 2016;36:594–603.
Chen J, Alemao E, Yin D, Cook J. Development of a diabetes treatment simulation model: with application to assessing alternative treatment intensification strategies on survival and diabetes-related complications. Diabetes Obes Metab. 2008;10(Suppl. 1):33–42.
Fabian MP, Stout NK, Adamkiewicz G, Geggel A, Ren C, Sandel M, et al. The effects of indoor environmental exposures on pediatric asthma: a discrete event simulation model. Environ Health. 2012;11:66.
Batina NG, Trentham-Dietz A, Gangnon RE, Sprague BL, Rosenberg MA, Stout NK, et al. Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat. 2013;138:519–28.
Stout NK, Goldhaber-Fiebert JD, Ortendahl JD, Goldie SJ. Trade-offs in cervical cancer prevention: balancing benefits and risks. Arch Intern Med. 2008;168:1881–9.
Pfizer Inc. Lyrica [prescribing information]. 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed 31 Jan 2017.
Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–10.
Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253–60.
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–38.
Emir B, Johnson K, Kuhn M, Parsons B. Predictive modeling of response to pregabalin for the treatment of neuropathic pain using 6-week observational data: a spectrum of modern analytics applications. Clin Ther. 2017;39:98–106.
Iacus S, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20:1–24.
Alexander J, Edwards R, Brodsky M, Manca L, Grugni R, Savoldelli A, et al. Using time series analysis approaches for improved prediction of pain outcomes in subgroups of patients with painful diabetic peripheral neuropathy. PLoS One. 2018;13:e0207120.
Parsons B, Li C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathy. Curr Med Res Opin. 2016;32:929–37.
Vinik A, Emir B, Parsons B, Cheung R. Prediction of pregabalin-mediated pain response by severity of sleep disturbance in patients with painful diabetic neuropathy and post-herpetic neuralgia. Pain Med. 2014;15:661–70.
Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–14.
Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002.
Anastassiou E, Iatrou CA, Vlaikidis N, Vafiadou M, Stamatiou G, Plesia E, et al. Impact of pregabalin treatment on pain, pain-related sleep interference and general well-being in patients with neuropathic pain: a non-interventional, multicentre, post-marketing study. Clin Drug Investig. 2011;31:417–26.
Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31:1448–54.
Pérez C, Latymer M, Almas M, Ortiz M, Clair A, Parsons B, et al. Does duration of neuropathic pain impact the effectiveness of pregabalin? Pain Pract. 2017;17:470–9.
Raskin P, Huffman C, Toth C, Asmus MJ, Messig M, Sanchez RJ, et al. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trial. Clin J Pain. 2014;30:379–90.
Semel D, Murphy TK, Zlateva G, Cheung R, Emir B. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
Alexander J, Edwards RA, Brodsky M, Manca L, Grugni R, Savoldelli A, et al. Using time series analysis approaches for improved prediction of pain outcomes in subgroups of patients with painful diabetic peripheral neuropathy. PLoS One. 2018;13(12):e0207120.
Alexander J, Edwards RA, Savoldelli A, Manca L, Grugni R, Emir B, et al. Integrating data from randomized controlled trials and observational studies to predict the response to pregabalin in patients with painful diabetic peripheral neuropathy. BMC Med Res Methodol. 2017;17:113.
Acknowledgements
Editorial support in the form of copy editing and formatting was provided by Ray Beck, Jr, PhD, of Engage Scientific Solutions and was funded by Pfizer. These analyses were funded by Pfizer.
Author information
Authors and Affiliations
Contributions
RAE and JA conceived, designed, and led all aspects of the analyses and related article. LM, AS, and RG performed all statistical analyses and/or simulation/analytics related to this study. EW and BE performed the statistical analyses for the original randomized controlled trials and observational study and offered insights related to those studies and analyses. BP, MB, and SW provided interpretations of the data related to clinical relevance and unmet medical needs. All authors participated in the drafting of the article and final approval of its content.
Corresponding author
Ethics declarations
Funding
These analyses were funded by Pfizer.
Conflict of interest
BE, BP, SW, and EW are employees of Pfizer. JA and MB were employed by Pfizer at the time the study was conducted. RE is an employee of Health Services Consulting Corporation who was a paid consultant by Pfizer in connection with this study and development of this article. LM, RG, and AS are employees of Fair Dynamics Consulting who were paid subcontractors to Health Services Consulting Corporation in connection with this study and the development of this article.
Additional information
Joe Alexander Jr and Marina Brodsky are formerly affiliated with Pfizer Inc, New York, NY, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alexander, J., Edwards, R.A., Brodsky, M. et al. Assessing the Value of Time Series Real-World and Clinical Trial Data vs. Baseline-Only Data in Predicting Responses to Pregabalin Therapy for Patients with Painful Diabetic Peripheral Neuropathy. Clin Drug Investig 39, 775–786 (2019). https://doi.org/10.1007/s40261-019-00812-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00812-6