Abstract
Background and Objectives
The hematological side effects associated with mycophenolic acid (MPA) are relatively common and have severe consequences. The majority of literature data have not shown clear consistency in the MPA exposure-neutropenia relationship. We hypothesized that (i) adult de novo kidney transplant recipients who develop neutropenia have relatively higher dose-normalized MPA exposure than patients without neutropenia, and (ii) the observed neutropenia may be explained by polymorphisms in metabolism and/or transporter genes responsible for MPA disposition.
Methods
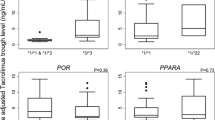
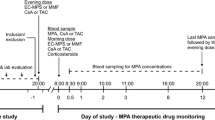
Adult kidney transplant recipients on steady-state tacrolimus and MPA, not receiving a corticosteroid, and with stable renal function were recruited for investigation at three periods post-transplant (1, 3, and 12 months; n = 21, 17, and 13, respectively). Clinical variables (age, weight, MPA daily dose, albumin, serum creatinine, absolute neutrophil count), tacrolimus and MPA concentrations (for exposure calculation), and genotypes (UGT2B7 G211T, UGT2B7 C802T, UGT1A9 T-275A, UGT1A9 T98C, MRP2 C-24T, MRP2 G1249A, OATP1B1 A388G, OATP1B1 C463A) were characterized.
Results
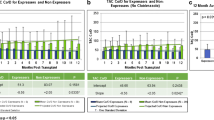
A significant inverse association between dose-normalized MPA exposure (a surrogate marker for apparent MPA clearance) and absolute neutrophil count in all three study periods (r2 ~ 0.3–0.7) was observed. No associations between characterized single nucleotide polymorphisms and MPA exposure or absolute neutrophil count were established. However, significant alterations in the minor allele frequencies of UGT2B7*2 C802T, UGT1A9 T275A, and MRP2 G1249A were evident.
Conclusion
These findings support the clinical strategy for conducting MPA therapeutic drug monitoring in adult kidney transplant patients on steroid-free immunosuppressant therapy. The novel population genomic analysis data warrant further epidemiological investigations in a larger study sample.




Similar content being viewed by others
References
Kiang TK, Ensom MH. Anti-rejection drugs. In: Murphy JE, editor. Clinical pharmacokinetics. 6th ed. American Society of Health-System Pharmacists: Bethesda; 2017. p. 205–20.
Kiang TK, Ensom MH. Immunosuppressants. In: Beringer PE, editor. Basic clinical pharmacokinetics. 6th ed. South Holland: Wolters Kluwer; 2017. p. 320–58.
Lemieux I, Houde I, Pascot A, Lachance JG, Noel R, Radeau T, Despres JP, Bergeron J. Effects of prednisone withdrawal on the new metabolic triad in cyclosporine-treated kidney transplant patients. Kidney Int. 2002;62(5):1839–47.
Andrade-Sierra J, Rojas-Campos E, Cardona-Munoz E, Evangelista-Carrillo LA, Puentes-Camacho A, Lugo-Lopez O, Gomez B, Valdespino C, Cerrillos I, Medina-Perez M, Jalomo B, Nieves JJ, Sandoval M, Ramos-Solano F, Monteon-Ramos F, Cueto-Manzano AM. Early steroid withdrawal in a renal transplant cohort treated with tacrolimus, mycophenolate mofetil and basiliximab. Nefrologia. 2014;34(2):216–22.
van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hene RJ, Verpooten GA, Navarro MT, Hale MD, Nicholls AJ. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68(2):261–6.
Kuypers DR, de Jonge H, Naesens M, de Loor H, Halewijck E, Dekens M, Vanrenterghem Y. Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther. 2008;30(4):673–83.
Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88(7):1351–89.
Barraclough KA, Lee KJ, Staatz CE. Pharmacogenetic influences on mycophenolate therapy. Pharmacogenomics. 2010;11(3):369–90.
Poulin E, Greanya ED, Partovi N, Shapiro RJ, Al-Khatib M, Ensom MH. Development and validation of limited sampling strategies for tacrolimus and mycophenolate in steroid-free renal transplant regimens. Ther Drug Monit. 2011;33(1):50–5.
de Winter BC, Mathot RA, Sombogaard F, Vulto AG, van Gelder T. Nonlinear relationship between mycophenolate mofetil dose and mycophenolic acid exposure: implications for therapeutic drug monitoring. Clin J Am Soc Nephrol. 2011;6(3):656–63.
Vancouver General Hospital Department of Pathology and Laboratory Medicine. Standard operating procedure: immunosuppressants. SOP. 2007;14768C:1–13.
Kim JY, Cheong HS, Park BL, Kim LH, Namgoong S, Kim JO, Kim HD, Kim YH, Chung MW, Han SY, Shin HD. Comprehensive variant screening of the UGT gene family. Yonsei Med J. 2014;55(1):232–9.
Johnson LA, Oetting WS, Basu S, Prausa S, Matas A, Jacobson PA. Pharmacogenetic effect of the UGT polymorphisms on mycophenolate is modified by calcineurin inhibitors. Eur J Clin Pharmacol. 2008;64(11):1047–56.
Zakerska O, Skrzypczak-Zielinska M, Mikstacki A, Tamowicz B, Malengowska B, Szalata M, Slomski R. Genotype and allele frequencies of polymorphic UGT1A9 in the Polish population. Eur J Drug Metab Pharmacokinet. 2013;38(3):217–21.
van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Schmidt J, Budde K, Kuypers D, Le Meur Y, van der Werf M, Mamelok R, van Gelder T. UGT1A9 -275T > A/-2152C > T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86(3):319–27.
Levesque E, Delage R, Benoit-Biancamano MO, Caron P, Bernard O, Couture F, Guillemette C. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81(3):392–400.
Edavana VK, Penney RB, Yao-Borengasser A, Starlard-Davenport A, Dhakal IB, Kadlubar S. Effect of MRP2 and MRP3 polymorphisms on anastrozole glucuronidation and MRP2 and MRP3 gene expression in normal liver samples. Int J Cancer Res Mol Mech. 2015. https://doi.org/10.16966/2381-3318.112.
Ho WF, Koo SH, Yee JY, Lee JD. Genetic variations of the ABCC2 gene in the Chinese, Malay, and Indian populations of Singapore. Drug Metab Pharmacokinet. 2008;23(5):385–91.
Peng KW, Bacon J, Zheng M, Guo Y, Wang MZ. Ethnic variability in the expression of hepatic drug transporters: absolute quantification by an optimized targeted quantitative proteomic approach. Drug Metab Dispos. 2015;43(7):1045–55.
Zhang X, Pu Z, Ge J, Shen J, Yuan X, Xie H. Association of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C polymorphisms and overall survival of breast cancer patients after tamoxifen therapy. Med Sci Monit. 2015;21:563–9.
Giacomini KM, Balimane PV, Cho SK, Eadon M, Edeki T, Hillgren KM, Huang SM, Sugiyama Y, Weitz D, Wen Y, Xia CQ, Yee SW, Zimdahl H, Niemi M. International Transporter Consortium. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin Pharmacol Ther. 2013;94(1):23–6.
Zhai X, Wang H, Zhu X, Miao H, Qian X, Li J, Gao Y, Lu F, Wu Y. Gene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemia. Arch Med Sci. 2012;8(4):659–71.
Olson KC, Dellinger RW, Zhong Q, Sun D, Amin S, Spratt TE, Lazarus P. Functional characterization of low-prevalence missense polymorphisms in the UDP-glucuronosyltransferase 1A9 gene. Drug Metab Dispos. 2009;37(10):1999–2007.
Deng XY, Wang CX, Wang XD, Bi HC, Chen X, Li JL, Huang M. Genetic polymorphisms of UGT1A8, UGT1A9, UGT2B7 and ABCC2 in Chinese renal transplant recipients and a comparison with other ethnic populations. Pharmazie. 2013;68(4):240–4.
Ramesh K, Hemanth Kumar AK, Kannan T, Vijayalakshmi R, Sudha V, Manohar Nesakumar S, Bharathiraja T, Lavanya J, Swaminathan S, Ramachandran G. SLCO1B1 gene polymorphisms do not influence plasma rifampicin concentrations in a South Indian population. Int J Tuberc Lung Dis. 2016;20(9):1231–5.
Lu XF, Zhou Y, Bi KS, Chen XH. Mixed effects of OATP1B1, BCRP and NTCP polymorphisms on the population pharmacokinetics of pravastatin in healthy volunteers. Xenobiotica. 2016;46(9):841–9.
Hurst FP, Belur P, Nee R, Agodoa LY, Patel P, Abbott KC, Jindal RM. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92(1):36–40.
Zafrani L, Truffaut L, Kreis H, Etienne D, Rafat C, Lechaton S, Anglicheau D, Zuber J, Ciroldi M, Thervet E, Snanoudj R, Mamzer MF, Martinez F, Timsit MO, Bergougnoux L, Legendre C. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009;9(8):1816–25.
Le Meur Y, Borrows R, Pescovitz MD, Budde K, Grinyo J, Bloom R, Gaston R, Walker RG, Kuypers D, van Gelder T, Kiberd B. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of the Transplantation Society consensus meeting. Transplant Rev (Orlando). 2011;25(2):58–64.
Vanhove T, Kuypers D, Claes KJ, Evenepoel P, Meijers B, Naesens M, Vanrenterghem Y, Cornelis T, Bammens B. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26(8):813–21.
Lam S, Partovi N, Ting LS, Ensom MH. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42(7):1037–47.
Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773–8.
Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, Ostrowski M, Fauchald P, Kokot F, Stefoni S, Perner F, Claesson K, Castagneto M, Heemann U, Carmellini M, Squifflet JP, Weber M, Segoloni G, Backman L, Sperschneider H, Kramer BK. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005;80(12):1734–41.
Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, FREEDOM Study Group. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–16.
Mourad M, Malaise J, Chaib Eddour D, De Meyer M, Konig J, Schepers R, Squifflet JP, Wallemacq P. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. 2001;47(1):88–94.
Atcheson BA, Taylor PJ, Mudge DW, Johnson DW, Hawley CM, Campbell SB, Isbel NM, Pillans PI, Tett SE. Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant. Br J Clin Pharmacol. 2005;59(3):271–80.
Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75(5):434–47.
Kiang TK, Ensom MH. Therapeutic drug monitoring of mycophenolate in adult solid organ transplant patients: an update. Expert Opin Drug Metab Toxicol. 2016;12(5):545–53.
De Rycke A, Dierickx D, Kuypers DR. Tacrolimus-induced neutropenia in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(3):690–4.
Shihab FS, Olyaei A, Wiland A, McCague K, Norman DJ. Tacrolimus exposure in the real world: an analysis from the Mycophenolic acid Observational REnal transplant study. Clin Transplant. 2014;28(7):768–75.
Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther. 2005;78(4):351–61.
Woillard JB, Picard N, Thierry A, Touchard G, Marquet P, DOMINOS Study Group. Associations between polymorphisms in target, metabolism, or transport proteins of mycophenolate sodium and therapeutic or adverse effects in kidney transplant patients. Pharmacogenet Genomics. 2014;24(5):256–62.
Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA, Haufroid V. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monit. 2016;38(Suppl 1):S57–69.
Acknowledgements
We sincerely thank Trana Hussaini for assistance in patient recruitment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by an unrestricted educational grant from Hoffmann-La Roche Limited (principal investigator: Mary H.H. Ensom) and the 2015 Vancouver Coastal Health Research Institute Team Grant Award (principal investigator: Tony K.L. Kiang). No editorial assistance was received for the preparation of this article.
Conflicts of interest
Nilufar Partovi, R. Jean Shapiro, Jacob M. Berman, and Abby Collier report no conflicts of interest related to the publication of this article. Tony K.L. Kiang and Mary H.H. Ensom held grant supports as detailed above (which did not pose a conflict of interest with this article), and do not report any other conflicts of interest.
Ethics Approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments). Ethics and operation approval were obtained from the University of British Columbia Clinical Research Ethics Board and Vancouver Coastal Health Research Institute, respectively.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Data Source
Original data can be made available upon request to the corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiang, T.K.L., Partovi, N., Shapiro, R.J. et al. Regression and Genomic Analyses on the Association Between Dose-Normalized Mycophenolic Acid Exposure and Absolute Neutrophil Count in Steroid-Free, De Novo Kidney Transplant Recipients. Clin Drug Investig 38, 1011–1022 (2018). https://doi.org/10.1007/s40261-018-0694-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0694-5