Abstract
Background and Objective
Highly active antiretroviral therapy (HAART) has modified the clinical course of human immunodeficiency virus (HIV) infection, reducing the rate of disease progression, the incidence of opportunistic infections and mortality. Several recent studies show early antiretroviral therapy reduces the risk of AIDS and HIV-related disease. The aim of this study was to perform an economic analysis to estimate the cost-utility of early antiretroviral therapy in Italy for managing HIV-infected patients.
Methods
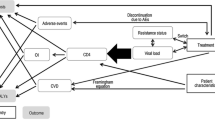
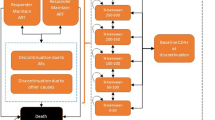
The incremental cost-utility analysis was carried out to quantify the benefits of the early-treatment approach in HIV subjects. A Markov simulation model including direct costs and health outcomes was developed from a third-party (Italian National Healthcare Service) payer’s perspective for four CD4 strata. 5000 Monte Carlo simulations were performed on two distinct scenarios: Standard of care (SoC) in which 30 % of patients started HAART with a CD4 count ≥500 cells/mm3 versus the early-treatment scenario (ETS), where the number of patients starting HAART with a CD4 count ≥500 cells/mm3 increased to 70 %. A systematic literature review was carried out to identify epidemiological and economic data, which were subsequently used to inform the model. In addition, a one-way probabilistic sensitivity analysis was performed in order to measure the relationship between the effectiveness of the treatments and the number of patients to undergo early treatment.
Results
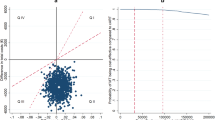
The model shows, in terms of the incremental cost-effectiveness ratio (ICER) per quality-adjusted life years (QALY) gained, that early treatment appeared to be the most cost-effective option compared to SoC (ICER = €13,625) over a time horizon of 10 years. The cost effectiveness of ETS is more sustainable as it extends the time horizon analysis (ICER = €7526 per QALY to 20 years and €8382 per QALY to 30 years). The one-way sensitivity analysis on the main variables confirmed the robustness of the model for the early-treatment approach.
Conclusion
Our model represents a tool for policy makers and health-care professionals to provide information on the cost effectiveness of the early-treatment approach in HIV-infected patients in Italy. Starting HAART earlier keeps HIV-infected patients in better health and reduces the incidence of AIDS- and non-AIDS-related events, generating a gain in terms of both patients’ health and correct resource allocation.




Similar content being viewed by others
References
European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2013. In: European Centre for Disease Prevention and Control; 2013.
National Institute for Health. Surveillance of new HIV infections diagnoses. Bull Natl Inst Health. 2014;27:6–16.
World Health Organization (WHO). Global update on the health sector response to HIV. 2014. http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf?ua=1. Accessed Jan 2016.
Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26.
Hiv-Causal Collaboration, Ray M, Logan R, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids. 2010;24:123–37.
Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505.
Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9.
Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. Aids. 2006;20:1447–50.
Rizzardini G, Restelli U, Bonfanti P, et al. The cost of HIV disease in Northern Italy: the payer’s perspective. J Acquir Immune Defic Syndr. 2011;57:211–7.
Rizzardini G, Bonfanti P, Carenzi L, et al. Cost-effectiveness analysis of HIV treatment in the clinical practice of a public hospital in northern Italy. Ther Clin Risk Manag. 2012;8:377–84.
Foglia E, Bonfanti P, Rizzardini G, et al. Cost-utility analysis of lopinavir/ritonavir versus atazanavir + ritonavir administered as first-line therapy for the treatment of HIV infection in Italy: from randomised trial to real world. PloS one. 2013;8:e57777.
Colombo GL, Colangeli V, Di Biagio A, et al. Cost-effectiveness analysis of initial HIV treatment under Italian guidelines. ClinicoEcon Outcomes Res CEOR. 2011;3:197–205.
Colombo GL, Di Matteo S, Antinori A, et al. Economic evaluation of initial antiretroviral therapy for HIV-infected patients: an update of Italian guidelines. ClinicoEcon Outcomes Res CEOR. 2013;5:489–96.
Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33.
Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:10–5.
Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64.
Italian Ministry of Health. Italian Guidelines on the use of antiretroviral drugs and the diagnostic-clinical management of HIV-1 infected people. 2012. http://www.salute.gov.it/imgs/c_17_pubblicazioni_2074_allegato.pdf. Accessed Jan 2016.
Masala G, Cannas G, Micocci M. Survival probabilities for HIV infected patients through semi-Markov processes. Biom Lett. 2014;51:13–36.
Centres for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR–17):1–19.
Mocroft A, Furrer HJ, Miro JM, et al. The incidence of AIDS-defining illnesses at a current CD4 count >/= 200 cells/muL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57:1038–47.
Simpson KN, Luo MP, Chumney E, et al. Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials. 2004;5:294–304.
Kauf TL, Roskell N, Shearer A, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. 2008;11:1144–53.
Brazier JRJ, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–92.
Work group on antiretroviral therapy of Lazio Region. Regional Diagnostic Therapeutic itinerary (PDT) on Antiretroviral Therapy, II Edition. In: http://www.regione.lazio.it/binary/rl_sanita/tbl_normativa/Decr_U00002_13_1_13_aggiornamento_terapia_HIV.pdf. Accessed Nov 2015.
Russo P. La Valutazione Framacoeconomica nel contesto regolatorio Italiano. Pharmacoeconomics Ital Res Articl. 2008;10:59–75.
Italian Health Economics Association (AIES). Guideline proposal for the economic evaluation of health intervention. Health Politics. 2009;10:91–9.
Favato G, Baio G, Capone A, et al. Novel health economic evaluation of a vaccination strategy to prevent HPV-related diseases: the BEST study. Med care. 2012;50:1076–85.
Drummond MF, Drummond MFMfteeohcp. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Drummond MF, McGuire A. Economic evaluation in health care : merging theory with practice. Oxford: Oxford University Press; 2001.
Briggs A, Claxton K, Sculpher M. Decision Modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Beck EJ. Modeling the cost-effectiveness of health programs: HIV testing and early treatment in the USA. Future Microbiol. 2011;6:725–9.
Beck EJ, Mandalia S, Sangha R, et al. The cost-effectiveness of early access to HIV services and starting cART in the UK 1996–2008. PloS one. 2011;6:e27830.
Koenig SP, Bang H, Severe P, et al. Cost-effectiveness of early versus standard antiretroviral therapy in HIV-infected adults in Haiti. PLoS Med. 2011;8:e1001095.
Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54.
Mellors JW, Rinaldo CR Jr, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70.
O’Brien WA, Hartigan PM, Daar ES, et al. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. VA Cooperative Study Group on AIDS. Anna Intern Med. 1997;126:939–45.
Lange JM. Current HIV clinical trial design issues. J Acquir Immune Defic Syndr Human Retrovirol. 1995;10(Suppl 1):S47–51.
Gilbert PB, DeGruttola V, Hammer SM, et al. Virologic and regimen termination surrogate end points in AIDS clinical trials. JAMA. 2001;285:777–84.
Acknowledgments
This study was realized by ARHEA S.r.l. within the project “Early treatment in HIV patients: a cost-utility analysis from the Italian perspective” for Janssen-Cilag Italy. The authors thank Francesco Damele, Lucia Mazzamuto and Daniela Mancusi (Janssen-Cilag Italy) for their invaluable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
The study was supported by an unrestricted fund from Janssen-Cilag, Italy.
Rights and permissions
About this article
Cite this article
Marcellusi, A., Viti, R., Russo, S. et al. Early Treatment in HIV Patients: A Cost–Utility Analysis from the Italian Perspective. Clin Drug Investig 36, 377–387 (2016). https://doi.org/10.1007/s40261-016-0382-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0382-2