Abstract
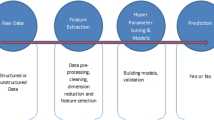
In recent years, machine learning (ML) techniques have garnered considerable interest for their potential use in accelerating the rate of drug discovery. With the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, the utilization of ML has become even more crucial in the search for effective antiviral medications. The pandemic has presented the scientific community with a unique challenge, and the rapid identification of potential treatments has become an urgent priority. Researchers have been able to accelerate the process of identifying drug candidates, repurposing existing drugs, and designing new compounds with desirable properties using machine learning in drug discovery. To train predictive models, ML techniques in drug discovery rely on the analysis of large datasets, including both experimental and clinical data. These models can be used to predict the biological activities, potential side effects, and interactions with specific target proteins of drug candidates. This strategy has proven to be an effective method for identifying potential coronavirus disease 2019 (COVID-19) and other disease treatments. This paper offers a thorough analysis of the various ML techniques implemented to combat COVID-19, including supervised and unsupervised learning, deep learning, and natural language processing. The paper discusses the impact of these techniques on pandemic drug development, including the identification of potential treatments, the understanding of the disease mechanism, and the creation of effective and safe therapeutics. The lessons learned can be applied to future outbreaks and drug discovery initiatives.










Similar content being viewed by others
References
Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–302.
Richards F, Kodjamanova P, Chen X, Li N, Atanasov P, Bennetts L, Patterson BJ, Yektashenas B, Mesa-Frias M, Tronczynski K. Economic burden of COVID-19: a systematic review. Clinicoecon Outcomes Res CEOR. 2022;14:293.
Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127: 104362.
Nicholls J, Dong XP, Jiang G, Peiris M. SARS: clinical virology and pathogenesis. Respirology. 2003;8:S6–8.
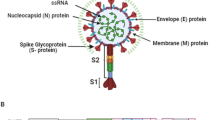
Troyano-Hernáez P, Reinosa R, Holguín Á. Evolution of SARS-CoV-2 envelope, membrane, nucleocapsid, and spike structural proteins from the beginning of the pandemic to September 2020: a global and regional approach by epidemiological week. Viruses. 2021;13(2):243.
V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–70.
Bai C, Zhong Q, Gao GF. Overview of SARS-CoV-2 genome-encoded proteins. Sci China Life Sci. 2022;65(2):280–94.
Zhou T, Tsybovsky Y, Gorman J, Rapp M, Cerutti G, Chuang G-Y, Katsamba PS, Sampson JM, Schön A, Bimela J. Cryo-EM structures of SARS-CoV-2 spike without and with ACE2 reveal a pH-dependent switch to mediate endosomal positioning of receptor-binding domains. Cell Host Microbe. 2020;28(6):867.e865-879.e865.
Nguyen HL, Lan PD, Thai NQ, Nissley DA, O’Brien EP, Li MS. Does SARS-CoV-2 bind to human ACE2 more strongly than does SARS-CoV? J Phys Chem B. 2020;124(34):7336–47.
Domingo P, Mur I, Pomar V, Corominas H, Casademont J, de Benito N. The four horsemen of a viral Apocalypse: the pathogenesis of SARS-CoV-2 infection (COVID-19). EBioMedicine. 2020;58: 102887.
Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
Bulut C, Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50(9):563–70.
Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J: The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;20(3):318–31.
Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–60.
Lavecchia A. Machine-learning approaches in drug discovery: methods and applications. Drug Discov Today. 2015;20(3):318–31.
Lo Y-C, Rensi SE, Torng W, Altman RB. Machine learning in chemoinformatics and drug discovery. Drug Discov Today. 2018;23(8):1538–46.
Kim E, Choi A-S, Nam H. Drug repositioning of herbal compounds via a machine-learning approach. BMC Bioinform. 2019;20(10):33–43.
Vo AH, Van Vleet TR, Gupta RR, Liguori MJ, Rao MS. An overview of machine learning and big data for drug toxicity evaluation. Chem Res Toxicol. 2019;33(1):20–37.
Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G, Li B, Madabhushi A, Shah P, Spitzer M. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov. 2019;18(6):463–77.
Kowalewski J, Ray A. Predicting novel drugs for SARS-CoV-2 using machine learning from a > 10 million chemical space. Heliyon. 2020;6(8):e04639.
Pham TH, Qiu Y, Zeng JC, Xie L, Zhang P. A deep learning framework for high-throughput mechanism-driven phenotype compound screening and its application to COVID-19 drug repurposing. Nat Mach Intell. 2021;3(3):247–57.
El-Behery H, Attia AF, El-Feshawy N, Torkey H. Efficient machine learning model for predicting drug-target interactions with case study for Covid-19. Comput Biol Chem. 2021;93:107536.
Lv H, Shi L, Berkenpas JW, Dao FY, Zulfiqar H, Ding H, Zhang Y, Yang LM, Cao RZ. Application of artificial intelligence and machine learning for COVID-19 drug discovery and vaccine design. Brief Bioinform. 2021;1–10:bbab320. https://doi.org/10.1093/bib/bbab320.
Liu Y, Gan J, Wang R, Yang X, Xiao Z, Cao Y. DrugDevCovid19: an atlas of anti-COVID-19 compounds derived by computer-aided drug design. Molecules. 2022;27(3):683.
Gupta A, Müller AT, Huisman BJH, Fuchs JA, Schneider P, Schneider G. Generative recurrent networks for de novo drug design. Mol Inf. 2018;37(1–2):1700111.
Zhang L, Zhang H, Ai H, Hu H, Li S, Zhao J, Liu H. Applications of machine learning methods in drug toxicity prediction. Curr Top Med Chem. 2018;18(12):987–97.
White J. PubMed 2.0. Med Ref Serv Q. 2020;39(4):382–7.
Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, Wang J, Williams R, Trawick BW, Pruitt KD, Sherry ST. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022;50(D1):D20–6. https://doi.org/10.1093/nar/gkab1112.
Zhang L, Tan J, Han D, Zhu H. From machine learning to deep learning: progress in machine intelligence for rational drug discovery. Drug Discov Today. 2017;22(11):1680–5.
Ekins S, Puhl AC, Zorn KM, Lane TR, Russo DP, Klein JJ, Hickey AJ, Clark AM. Exploiting machine learning for end-to-end drug discovery and development. Nat Mater. 2019;18(5):435–41.
Patel L, Shukla T, Huang X, Ussery DW, Wang S. Machine learning methods in drug discovery. Molecules. 2020;25(22):5277.
Castillo TJM, Arif M, Niessen WJ, Schoots IG, Veenland JF. Automated classification of significant prostate cancer on MRI: a systematic review on the performance of machine learning applications. Cancers (Basel). 2020;12(6):1606.
Cuocolo R, Cipullo MB, Stanzione A, Romeo V, Green R, Cantoni V, Ponsiglione A, Ugga L, Imbriaco M. Machine learning for the identification of clinically significant prostate cancer on MRI: a meta-analysis. Eur Radiol. 2020;30:6877–87.
Cuocolo R, Cipullo MB, Stanzione A, Ugga L, Romeo V, Radice L, Brunetti A, Imbriaco M. Machine learning applications in prostate cancer magnetic resonance imaging. Eur Radiol Exp. 2019;3(1):1–8.
Yang H, Sun L, Li W, Liu G, Tang Y. In silico prediction of chemical toxicity for drug design using machine learning methods and structural alerts. Front Chem. 2018;6:30.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Sinaga KP, Yang M-S. Unsupervised K-means clustering algorithm. IEEE Access. 2020;8:80716–27.
Kodinariya TM, Makwana PR. Review on determining number of cluster in K-means clustering. Int J. 2013;1(6):90–5.
Pham DT, Dimov SS, Nguyen CD. Selection of K in K-means clustering. Proc Inst Mech Eng C J Mech Eng Sci. 2005;219(1):103–19.
Likas A, Vlassis N, Verbeek JJ. The global k-means clustering algorithm. Pattern Recogn. 2003;36(2):451–61.
Hartigan JA, Wong MA. Algorithm AS 136: a k-means clustering algorithm. J R Stat Soc Ser C (Appl Stat). 1979;28(1):100–8.
Golmohammadi H, Dashtbozorgi Z, Acree WE Jr. Quantitative structure–activity relationship prediction of blood-to-brain partitioning behavior using support vector machine. Eur J Pharm Sci. 2012;47(2):421–9.
Shar PA, Tao W, Gao S, Huang C, Li B, Zhang W, Shahen M, Zheng C, Bai Y, Wang Y. Pred-binding: large-scale protein–ligand binding affinity prediction. J Enzyme Inhib Med Chem. 2016;31(6):1443–50.
Zsila F, Bikadi Z, Malik D, Hari P, Pechan I, Berces A, Hazai E. Evaluation of drug–human serum albumin binding interactions with support vector machine aided online automated docking. Bioinformatics. 2011;27(13):1806–13.
Passerini A, Punta M, Ceroni A, Rost B, Frasconi P. Identifying cysteines and histidines in transition-metal-binding sites using support vector machines and neural networks. Proteins Struct Funct Bioinform. 2006;65(2):305–16.
Cai Y-D, Lin SL. Support vector machines for predicting rRNA-, RNA-, and DNA-binding proteins from amino acid sequence. Biochim Biophys Acta (BBA) Proteins Proteom. 2003;1648(1–2):127–33.
Bradford JR, Westhead DR. Improved prediction of protein–protein binding sites using a support vector machines approach. Bioinformatics. 2005;21(8):1487–94.
Ali J, Khan R, Ahmad N, Maqsood I. Random forests and decision trees. Int J Comput Sci Issues (IJCSI). 2012;9(5):272.
Denisko D, Hoffman MM. Classification and interaction in random forests. Proc Natl Acad Sci. 2018;115(8):1690–2.
Fratello M, Tagliaferri R. Decision trees and random forests. Encyclopedia of bioinformatics and computational biology. 2019;1:374–83.
Shi H, Liu S, Chen J, Li X, Ma Q, Yu B. Predicting drug–target interactions using Lasso with random forest based on evolutionary information and chemical structure. Genomics. 2019;111(6):1839–52.
Bishop CM. Neural networks and their applications. Rev Sci Instrum. 1994;65(6):1803–32.
Abdi H. A neural network primer. J Biol Syst. 1994;2(03):247–81.
Agostinelli F, Hoffman M, Sadowski P, Baldi P. Learning activation functions to improve deep neural networks. arXiv preprint arXiv:14126830 2014.
Rasamoelina AD, Adjailia F, Sinčák P. A review of activation function for artificial neural network. In: 2020 IEEE 18th World symposium on applied machine intelligence and informatics (SAMI). IEEE. 2020. p. 281–6.
Yuen B, Hoang MT, Dong X, Lu T. Universal activation function for machine learning. Sci Rep. 2021;11(1):18757.
Zhang Z, Beck MW, Winkler DA, Huang B, Sibanda W, Goyal H. Opening the black box of neural networks: methods for interpreting neural network models in clinical applications. Ann Transl Med. 2018;6(11):216.
Dayhoff JE, DeLeo JM. Artificial neural networks: opening the black box. Cancer Interdiscipl Int J Am Cancer Soc. 2001;91(S8):1615–35.
Benítez JM, Castro JL, Requena I. Are artificial neural networks black boxes? IEEE Trans Neural Netw. 1997;8(5):1156–64.
Temurtas H, Yumusak N, Temurtas F. A comparative study on diabetes disease diagnosis using neural networks. Expert Syst Appl. 2009;36(4):8610–5.
Khemphila A, Boonjing V. Heart disease classification using neural network and feature selection. In: 2011 21st international conference on systems engineering: 16–18 Aug 2011. 2011. p. 406–9.
Sadighpour L, Rezaei S, Paknejad M, Jafary F, Aslani P. The application of an artificial neural network to support decision making in edentulous maxillary implant prostheses. J Res Pract Dent. 2014:2014:369025. https://doi.org/10.5171/2014.369025.
Jung S-K, Kim T-W. New approach for the diagnosis of extractions with neural network machine learning. Am J Orthod Dentofac Orthop. 2016;149(1):127–33.
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O. Deep learning with convolutional neural network in radiology. Jpn J Radiol. 2018;36:257–72.
de Souza JG, Fernandes MAC, Barbosa RD. A novel deep neural network technique for drug–target interaction. Pharmaceutics. 2022;14(3):625.
Karnati M, Seal A, Sahu G, Yazidi A, Krejcar O. A novel multi-scale based deep convolutional neural network for detecting COVID-19 from X-rays. Appl Soft Comput. 2022;125:109109.
Andrade CH, Pasqualoto KF, Ferreira EI, Hopfinger AJ. 4D-QSAR: perspectives in drug design. Molecules. 2010;15(5):3281–94.
Hopfinger A, Wang S, Tokarski JS, Jin B, Albuquerque M, Madhav PJ, Duraiswami C. Construction of 3D-QSAR models using the 4D-QSAR analysis formalism. J Am Chem Soc. 1997;119(43):10509–24.
Potemkin V, Grishina M. Principles for 3D/4D QSAR classification of drugs. Drug Discov Today. 2008;13(21–22):952–9.
Myshkin E, Brennan R, Khasanova T, Sitnik T, Serebriyskaya T, Litvinova E, Guryanov A, Nikolsky Y, Nikolskaya T, Bureeva S. Prediction of organ toxicity endpoints by qsar modeling based on precise chemical-histopathology annotations. Chem Bioogyl Drug Des. 2012;80(3):406–16.
Yang L, Wang Y, Chang J, Pan Y, Wei R, Li J, Wang H. QSAR modeling the toxicity of pesticides against Americamysis bahia. Chemosphere. 2020;258: 127217.
Klüver N, Vogs C, Altenburger R, Escher BI, Scholz S. Development of a general baseline toxicity QSAR model for the fish embryo acute toxicity test. Chemosphere. 2016;164:164–73.
Pavan M, Netzeva T, Worth A. Validation of a QSAR model for acute toxicity. SAR QSAR Environ Res. 2006;17(02):147–71.
Huang S-H, Tung C-W, Fülöp F, Li J-H. Developing a QSAR model for hepatotoxicity screening of the active compounds in traditional Chinese medicines. Food Chem Toxicol. 2015;78:71–7.
Rodgers AD, Zhu H, Fourches D, Rusyn I, Tropsha A. Modeling liver-related adverse effects of drugs using k nearest neighbor quantitative structure–activity relationship method. Chem Res Toxicol. 2010;23(4):724–32.
Fjodorova N, Vračko M, Novič M, Roncaglioni A, Benfenati E. New public QSAR model for carcinogenicity. In: Chemistry central journal: 2010. Springer. p. 1–15.
Rogers D, Hahn M. Extended-connectivity fingerprints. J Chem Inf Model. 2010;50(5):742–54.
Cereto-Massagué A, Ojeda MJ, Valls C, Mulero M, Garcia-Vallvé S, Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2015;71:58–63.
Kearnes S, McCloskey K, Berndl M, Pande V, Riley P. Molecular graph convolutions: moving beyond fingerprints. J Comput Aided Mol Des. 2016;30:595–608.
Pattanaik L, Coley CW. Molecular representation: going long on fingerprints. Chemistry. 2020;6(6):1204–7.
Aliper A, Plis S, Artemov A, Ulloa A, Mamoshina P, Zhavoronkov A. Deep learning applications for predicting pharmacological properties of drugs and drug repurposing using transcriptomic data. Mol Pharm. 2016;13(7):2524–30.
March-Vila E, Pinzi L, Sturm N, Tinivella A, Engkvist O, Chen HM, Rastelli G: On the integration of in silico drug design methods for drug repurposing. Front Pharmacol. 2017;8:298. https://doi.org/10.3389/fphar.2017.00298.
Wen M, Zhang ZM, Niu SY, Sha HZ, Yang RH, Yun YH, Lu HM. Deep-learning-based drug–target interaction prediction. J Proteome Res. 2017;16(4):1401–9.
Chang Y, Park H, Yang HJ, Lee S, Lee KY, Kim TS, Jung J, Shin JM. Cancer drug response profile scan (CDRscan): a deep learning model that predicts drug effectiveness from cancer genomic signature. Sci Rep. 2018;8(1):8857. https://doi.org/10.1038/s41598-018-27214-6.
Wan FP, Hong LX, Xiao A, Jiang T, Zeng JY. NeoDTI: neural integration of neighbor information from a heterogeneous network for discovering new drug-target interactions. Bioinformatics. 2019;35(1):104–11.
Zeng XX, Zhu SY, Liu XR, Zhou YD, Nussinov R, Cheng FX. DeepDR: a network-based deep learning approach to in silico drug repositioning. Bioinformatics. 2019;35(24):5191–8.
Zeng XX, Song X, Ma TF, Pan XQ, Zhou YD, Hou Y, Zhang Z, Li KL, Karypis G, Cheng FX. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J Proteome Res. 2020;19(11):4624–36.
Gysi DM, do Valle I, Zitnik M, Ameli A, Gan X, Varol O, Ghiassian SD, Patten JJ, Davey RA, Loscalzo J et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proced Natl Acad Sci USA 2021;118(19):e2025581118.
Santos SD, Torres M, Galeano D, Sanchez MD, Cernuzzi L, Paccanaro A. Machine learning and network medicine approaches for drug repositioning for COVID-19. Patterns. 2022;3(1):100396.
Ge YY, Tian TZ, Huang SL, Wan FP, Li JX, Li SY, Wang XT, Yang H, Hong LX, Wu N et al: An integrative drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. Signal Transduct Target Ther. 2021;6(1):165.
Smith DP, Oechsle O, Rawling MJ, Savory E, Lacoste A, Richardson PJ. Expert-augmented computational drug repurposing identified baricitinib as a treatment for COVID-19. Front Pharmacol. 2021;12:1699.
Kumar A, Loharch S, Kumar S, Ringe RP, Parkesh R. Exploiting cheminformatic and machine learning to navigate the available chemical space of potential small molecule inhibitors of SARS-CoV-2. Comput Struct Biotechnol J. 2021;19:424–38.
Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–90.
Gawriljuk VO, Zin PPK, Puhl AC, Zorn KM, Foil DH, Lane TR, Hurst B, Tavella TA, Costa FTM, Lakshmanane P, et al. Machine learning models identify inhibitors of SARS-CoV-2. J Chem Inf Model. 2021;61(9):4224–35.
Kadioglu O, Saeed M, Greten HJ, Efferth T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Comput Biol Med. 2021;133:104359.
Jiang X, Neapolitan RE. Mining pure, strict epistatic interactions from high-dimensional datasets: ameliorating the curse of dimensionality. PLoS One. 2012; 7(10):e467712012.
Chattopadhyay A, Lu T-P. Gene-gene interaction: the curse of dimensionality. Ann Transl Med. 2019;7(24):813.
Carse B, Fogarty TC: Tackling the “curse of dimensionality” of radial basis functional neural networks using a genetic algorithm. In: Parallel Problem solving from nature—PPSN IV: international conference on evolutionary computation—the 4th international conference on parallel problem solving from Nature Berlin, Germany, September 22–26, 1996 Proceedings 4: 1996. Springer. p. 707–19.
Fakoor R, Ladhak F, Nazi A, Huber M. using deep learning to enhance cancer diagnosis and classification. In: Proceedings of the ICML workshop on the role of machine learning in transforming healthcare (WHEALTH). Atlanta, GA. 2013.
Levine AB, Schlosser C, Grewal J, Coope R, Jones SJ, Yip S. Rise of the machines: advances in deep learning for cancer diagnosis. Trends Cancer. 2019;5(3):157–69.
Sun W, Zheng B, Qian W. Computer aided lung cancer diagnosis with deep learning algorithms. In: Medical imaging 2016: computer-aided diagnosis: 2016. SPIE: 241-248.
Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13(1):1–17.
Chen MC, Ball RL, Yang L, Moradzadeh N, Chapman BE, Larson DB, Langlotz CP, Amrhein TJ, Lungren MP. Deep learning to classify radiology free-text reports. Radiology. 2018;286(3):845–52.
Mazurowski MA, Buda M, Saha A, Bashir MR. Deep learning in radiology: an overview of the concepts and a survey of the state of the art with focus on MRI. J Magn Reson Imaging. 2019;49(4):939–54.
Wu R, Ding F, Wang R, Shen R, Zhang X, Luo S, Su C, Wu Z, Xie Q, Berger B. High-resolution de novo structure prediction from primary sequence. BioRxiv 2022:2022.2007. 2021.500999.
Wei L, Zou Q. Recent progress in machine learning-based methods for protein fold recognition. Int J Mol Sci. 2016;17(12):2118.
Wei G-W. Protein structure prediction beyond AlphaFold. Nat Mach Intell. 2019;1(8):336–7.
Noé F, De Fabritiis G, Clementi C. Machine learning for protein folding and dynamics. Curr Opin Struct Biol. 2020;60:77–84.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9.
Heo L, Feig M. High-accuracy protein structures by combining machine-learning with physics-based refinement. Proteins Struct Funct Bioinform. 2020;88(5):637–42.
Cheng J, Baldi P. A machine learning information retrieval approach to protein fold recognition. Bioinformatics. 2006;22(12):1456–63.
AlQuraishi M. Machine learning in protein structure prediction. Curr Opin Chem Biol. 2021;65:1–8.
Wang Z, Clark NR, Ma’ayan A. Drug-induced adverse events prediction with the LINCS L1000 data. Bioinformatics. 2016;32(15):2338–45.
Duan Q, Flynn C, Niepel M, Hafner M, Muhlich JL, Fernandez NF, Rouillard AD, Tan CM, Chen EY, Golub TR. LINCS Canvas Browser: interactive web app to query, browse and interrogate LINCS L1000 gene expression signatures. Nucleic Acids Res. 2014;42(W1):W449–60.
Zhang PL, Wei ZQ, Che C, Jin B. DeepMGT-DTI: transformer network incorporating multilayer graph information for drug–target interaction prediction. Comput Biol Med. 2022;142:105214.
Yazdani-Jahromi M, Yousefi N, Tayebi A, Kolanthai E, Neal CJ, Seal S, Garibay OO. AttentionSiteDTI: an interpretable graph-based model for drug-target interaction prediction using NLP sentence-level relation classification. Brief Bioinform. 2022;23(4):bbac272. https://doi.org/10.1093/bib/bbac272.
Yang YQ, Zhou DS, Zhang XB, Shi YL, Han JX, Zhou LP, Wu LY, Ma MF, Li JT, Peng SL et al. D3AI-CoV: a deep learning platform for predicting drug targets and for virtual screening against COVID-19. Brief Bioinform. 2022;23(3):bbac147. https://doi.org/10.1093/bib/bbac147.
Wei BM, Zhang Y, Gong X. DeepLPI: a novel deep learning-based model for protein–ligand interaction prediction for drug repurposing. Sci Rep. 2022;12(1):18200.
Wang SW, Sun Q, Xu YJ, Pei JF, Lai LH. A transferable deep learning approach to fast screen potential antiviral drugs against SARS-CoV-2. Brief Bioinform. 2021;22(6):bbab211.
Wang JJ, Wen NF, Wang CY, Zhao LL, Cheng L. ELECTRA-DTA: a new compound-protein binding affinity prediction model based on the contextualized sequence encoding. J Cheminform. 2022;14(1):1–14.
Timmons JA, Anighoro A, Brogan RJ, Stahl J, Wahlestedt C, Farquhar DG, Taylor-King J, Volmar CH, Kraus WE, Phillips SM. A human-based multi-gene signature enables quantitative drug repurposing for metabolic disease. Elife 2022;11:e68832.
Surianarayanan C, Chelliah PR. Leveraging artificial intelligence (AI) capabilities for COVID-19 containment. New Gener Comput. 2021;39(3–4):717–41.
Su XR, Hu L, You ZH, Hu PW, Wang L, Zhao BW. A deep learning method for repurposing antiviral drugs against new viruses via multi-view nonnegative matrix factorization and its application to SARS-CoV-2. Brief Bioinform. 2022;23(1):bbab526.
Silveira EC. Screening anti-inflammatory, anticoagulant, and respiratory agents for SARS-CoV-2 3CL(Pro) inhibition from chemical fingerprints through a deep learning approach. Clin Transl Investig. 2022;74(1):31–9.
Shorten C, Khoshgoftaar TM, Furht B. Deep learning applications for COVID-19. J Big Data. 2021;8:18.
Ray S, Lall S, Bandyopadhyay S. A deep integrated framework for predicting SARS-CoV2-human protein–protein interaction. IEEE Trans Emerg Top Comput Intell. 2022;6(6):1463–72.
Rajput A, Thakur A, Mukhopadhyay A, Kamboj S, Rastogi A, Gautam S, Jassal H, Kumar M. Prediction of repurposed drugs for Coronaviruses using artificial intelligence and machine learning. Comput Struct Biotechnol J. 2021;19:3133–48.
Pan XQ, Lin X, Cao DS, Zeng XX, Yu PS, He LF, Nussinov R, Cheng FX. Deep learning for drug repurposing: methods, databases, and applications. Wiley Interdiscip Rev Comput Mol Sci. 2022;12(4):e1597.
Moovarkumudalvan B, Geethakumari AM, Ramadoss R, Biswas KH, Mifsud B. Structure-based virtual screening and functional validation of potential hit molecules targeting the SARS-CoV-2 main protease. Biomolecules. 2022;12(12):1754.
Li ZR, Zhong Q, Yang J, Duan YJ, Wang WJ, Wu CK, He KL. DeepKG: an end-to-end deep learning-based workflow for biomedical knowledge graph extraction, optimization and applications. Bioinformatics. 2022;38(5):1477–9.
Lee CY, Chen YPP. New insights into drug repurposing for COVID-19 using deep learning. IEEE Trans Neural Netw Learn Syst. 2021;32(11):4770–80.
Kanapeckaite A, Mazeikiene A, Geris L, Burokiene N, Cottrell GS, Widera D. Computational pharmacology: new avenues for COVID-19 therapeutics search and better preparedness for future pandemic crises. Biophys Chem. 2022;290:106891.
Joshi T, Sharma P, Mathpal S, Joshi T, Maiti P, Nand M, Pande V, Chandra S. Computational investigation of drug bank compounds against 3C-like protease (3CL(pro)) of SARS-CoV-2 using deep learning and molecular dynamics simulation. Mol Divers. 2022;26(4):2243–56.
Hooshmand SA, Ghobadi MZ, Hooshmand SE, Jamalkandi SA, Alavi SM, Masoudi-Nejad A. A multimodal deep learning-based drug repurposing approach for treatment of COVID-19. Mol Divers. 2021;25(3):1717–30.
Harigua-Souiai E, Heinhane MM, Abdelkrim YZ, Souiai O, Abdeljaoued-Tej I, Guizani I. Deep learning algorithms achieved satisfactory predictions when trained on a novel collection of anticoronavirus molecules. Front Genet. 2021;12:744170.
Deepthi K, Jereesh AS, Liu YS. A deep learning ensemble approach to prioritize antiviral drugs against novel coronavirus SARS-CoV-2 for COVID-19 drug repurposing. Appl Soft Comput. 2021;113:107945.
Choi Y, Shin B, Kang K, Park S, Beck BR. Target-centered drug repurposing predictions of human angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine subtype 2 (TMPRSS2) interacting approved drugs for coronavirus disease 2019 (COVID-19) treatment through a drug–target interaction deep learning model. Viruses-Basel 2020;12(11):1325.
Anwaar MUU, Adnan F, Abro A, Khan RAA, Rehman AUU, Osama M, Rainville C, Kumar S, Sterner DEE, Javed S, et al. Combined deep learning and molecular docking simulations approach identifies potentially effective FDA approved drugs for repurposing against SARS-CoV-2. Comput Biol Med. 2022;141:105049.
Abdel-Basset M, Hawash H, Elhoseny M, Chakrabortty RK, Ryan M. DeepH-DTA: deep learning for predicting drug-target interactions: a case study of COVID-19 drug repurposing. IEEE Access. 2020;8:170433–51.
Percha B, Altman RB. A global network of biomedical relationships derived from text. Bioinformatics. 2018;34(15):2614–24.
Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V, et al. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42(1):D1091-1097.
Wang MY. Deep graph library: towards efficient and scalable deep learning on graphs. In: ICLR workshop on representation learning on graphs and manifolds. 2019.
Reis G, Silva EA, Silva DC, Thabane L, Milagres AC, Ferreira TS, Dos Santos CV, Campos VH, Nogueira AM, de Almeida AP. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med. 2022;386(18):1721–31.
Popp M, Stegemann M, Metzendorf M-I, Gould S, Kranke P, Meybohm P, Skoetz N, Weibel S. Ivermectin for preventing and treating COVID‐19. Cochrane Database Syst Rev. 2021;2021(7):CD015017. https://doi.org/10.1002/14651858.CD015017.pub2.
Torjesen I: Covid-19. Hydroxychloroquine does not benefit hospitalised patients, UK trial finds. BMJ Br Med J (Online). 2020;369:m2263.
Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022.
De Wilde AH, Falzarano D, Zevenhoven-Dobbe JC, Beugeling C, Fett C, Martellaro C, Posthuma CC, Feldmann H, Perlman S, Snijder EJ. Alisporivir inhibits MERS-and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13.
Softic L, Brillet R, Berry F, Ahnou N, Nevers Q, Morin-Dewaele M, Hamadat S, Bruscella P, Fourati S, Pawlotsky J-M. Inhibition of SARS-CoV-2 infection by the cyclophilin inhibitor alisporivir (Debio 025). Antimicrob Agents Chemother. 2020;64(7):e00876-e1820.
Pawlotsky J-M. COVID-19 pandemic: time to revive the cyclophilin inhibitor alisporivir. Clin Infect Dis. 2020;71(16):2191–4.
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807.
Rubin R. Baricitinib is first approved COVID-19 immunomodulatory treatment. JAMA. 2022;327(23):2281–2281.
Dunnivant FM, Elzerman AW, Jurs PC, Hasan MN. Quantitative structure property relationships for aqueous solubilities and henrys law constants of polychlorinated-biphenyls. Environ Sci Technol. 1992;26(8):1567–73.
Fisher SW, Lydy MJ, Barger J, Landrum PF. Quantitative structure–activity-relationships for predicting the toxicity of pesticides in aquatic systems with sediment. Environ Toxicol Chem. 1993;12(7):1307–18.
Karickhoff SW, McDaniel VK, Melton C, Vellino AN, Nute DE, Carreira LA. Predicting chemical-reactivity by computer. Environ Toxicol Chem. 1991;10(11):1405–16.
Moriguchi I, Hirono S, Liu Q, Matsushita Y, Nakagawa T. Fuzzy adaptive least-squares and its use in quantitative structure-activity-relationships. Chem Pharm Bull. 1990;38(12):3373–9.
Nabivach VM, Dmitrikov VP. Use of the correlation equations for the prediction of the retention data in gas–liquid-chromatography. Usp Khim. 1993;62(1):27–38.
Narayszabo G, Balogh T. The average molecular electrostatic-field as a QSAR descriptor. 4. hydrophobicity scales for amino-acid residues-alpha. J Mol Struct Theochem. 1993;103(3):243–8.
Stanton DT, Egolf LM, Jurs PC, Hicks MG. Computer-assisted prediction of normal boiling points of pyrans and pyrroles. J Chem Inf Comput Sci. 1992;32(4):306–16.
Suzuki T, Ohtaguchi K, Koide K. Correlation and prediction of autoignition temperatures of hydrocarbons using molecular-properties. J Chem Eng Jpn. 1992;25(5):606–8.
Cereto-Massague A, Ojeda MJ, Valls C, Mulero M, Garcia-Vallve S, Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2015;71:58–63.
Dong J, Cao DS, Miao HY, Liu S, Deng BC, Yun YH, Wang NN, Lu AP, Zeng WB, Chen AF. ChemDes: an integrated web-based platform for molecular descriptor and fingerprint computation. J Cheminform. 2015;7:1–10.
Kearnes S, McCloskey K, Berndl M, Pande V, Riley P. Molecular graph convolutions: moving beyond fingerprints. J Comput Aided Mol Des. 2016;30(8):595–608.
Yap CW. PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem. 2011;32(7):1466–74.
Russo DP, Zorn KM, Clark AM, Zhu H, Ekins S. Comparing multiple machine learning algorithms and metrics for estrogen receptor binding prediction. Mol Pharm. 2018;15(10):4361–70.
Chen B, Sheridan RP, Hornak V, Voigt JH. Comparison of random forest and pipeline pilot Naive Bayes in prospective QSAR predictions. J Chem Inf Model. 2012;52(3):792–803.
Lane TR, Foil DH, Minerali E, Urbina F, Zorn KM, Ekins S. Bioactivity comparison across multiple machine learning algorithms using over 5000 datasets for drug discovery. Mol Pharm. 2020;18(1):403–15.
Minerali E, Foil DH, Zorn KM, Ekins S. Evaluation of assay central machine learning models for rat acute oral toxicity prediction. ACS Sustain Chem Eng. 2020;8(42):16020–7.
Rosenke K, Jarvis MA, Feldmann F, Schwarz B, Okumura A, Lovaglio J, Saturday G, Hanley PW, Meade-White K, Williamson BN. Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS-CoV-2. BioRxiv. 2020.
Wang LL, Lo K, Chandrasekhar Y, Reas R, Yang J, Eide D, Funk K, Kinney R, Liu Z, Merrill W. Cord-19: the covid-19 open research dataset. arXiv. 2020.
Lo HS, Hui KPY, Lai H-M, He X, Khan KS, Kaur S, Huang J, Li Z, Chan AK, Cheung HH-Y. Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. ACS Cent Sci. 2021;7(5):792–802.
Muturi E, Hong W, Li J, Yang W, He J, Wei H, Yang H. Effects of simeprevir on the replication of SARS-CoV-2 in vitro and in transgenic hACE2 mice. Int J Antimicrob Agents. 2022;59(1): 106499.
Rahman MM, Saha T, Islam KJ, Suman RH, Biswas S, Rahat EU, Hossen MR, Islam R, Hossain MN, Mamun AA. Virtual screening, molecular dynamics and structure–activity relationship studies to identify potent approved drugs for Covid-19 treatment. J Biomol Struct Dyn. 2021;39(16):6231–41.
Abhithaj J, Dileep F, Sharanya C, Arun K, Sadasivan C, Jayadevi V. Repurposing simeprevir, calpain inhibitor IV and a cathepsin F inhibitor against SARS-CoV-2 and insights into their interactions with Mpro. J Biomol Struct Dyn. 2020;1:23–35.
Ahmed S, Mahtarin R, Ahmed SS, Akter S, Islam MS, Mamun AA, Islam R, Hossain MN, Ali MA, Sultana MU. Investigating the binding affinity, interaction, and structure–activity-relationship of 76 prescription antiviral drugs targeting RdRp and Mpro of SARS-CoV-2. J Biomol Struct Dyn. 2021;39(16):6290–305.
Shin B, Park S, Kang K, Ho JC: Self-attention based molecule representation for predicting drug-target interaction. In: Machine learning for healthcare conference: 2019. PMLR. p. 230–48.
Weininger D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J Chem Inf Comput Sci. 1988;28(1):31–6.
Pearson WR. Searching protein sequence libraries: comparison of the sensitivity and selectivity of the Smith–Waterman and FASTA algorithms. Genomics. 1991;11(3):635–50.
Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK. BindingDB: a web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res. 2007;35(suppl_1):D198–201.
Tanoli Z, Alam Z, Vähä-Koskela M, Ravikumar B, Malyutina A, Jaiswal A, Tang J, Wennerberg K, Aittokallio T: Drug Target Commons 2.0: a community platform for systematic analysis of drug–target interaction profiles. Database. 2018;2018:bay083. https://doi.org/10.1093/database/bay083.
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.
Kupferschmidt K, Wadman M: Delta variant triggers new phase in the pandemic. In: American Association for the Advancement of Science; 2021.
Christensen PA, Olsen RJ, Long SW, Subedi S, Davis JJ, Hodjat P, Walley DR, Kinskey JC, Saavedra MO, Pruitt L. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol. 2021;192(2):320–31.
CDC Statement on B. 1.1. 529 (Omicron variant), Media Statement Release on November 26, 2021. https://www.cdc.gov/media/releases/2021/s1126-B11-529-omicron.html.
Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. In. British Medical Journal Publishing Group; 2021.
Rao S, Singh M. The Newly Detected B. 1.1. 529 (Omicron) variant of SARS-CoV-2 With multiple mutations: implications for transmission, diagnostics, therapeutics, and immune evasion. DHR Proc. 2021;1(S5):7–10.
Sahoo JP, Samal KC. World on alert: WHO designated South African new COVID strain (Omicron/B. 1.1. 529) as a variant of concern. Biotica Res Today. 2021;3(11):1086–8.
Zhang X, Wu S, Wu B, Yang Q, Chen A, Li Y, Zhang Y, Pan T, Zhang H, He X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6(1):1–3.
Jahanshahlu L, Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother. 2020;129: 110337.
Clark SA, Clark LE, Pan J, Coscia A, McKay LG, Shankar S, Johnson RI, Griffiths A, Abraham J. Molecular basis for a germline-biased neutralizing antibody response to SARS-CoV-2. bioRxiv 2020.
Chen RE, Winkler ES, Case JB, Aziati ID, Bricker TL, Joshi A, Darling TL, Ying B, Errico JM, Shrihari S, VanBlargan LA. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596(7870):103–8.
Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD. Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7. Nature. 2021;593(7857):130–5.
Starr TN, Greaney AJ, Dingens AS, Bloom JD. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep Med. 2021;2(4): 100255.
Bertoglio F, Fühner V, Ruschig M, Heine PA, Abassi L, Klünemann T, Rand U, Meier D, Langreder N, Steinke S. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021;36(4): 109433.
Williams MA, Hall DR, Hulswit RJ, Bowden TA, Fry EE. Antibody evasion by the P. 1 strain of SARS-CoV-2. Cell. 2021;184:1–16.
Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland W-H, Porrot F, Staropoli I, Lemoine F. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–5.
Chen J, Wang R, Gilby NB, Wei G-W. Omicron variant (B. 1.1. 529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022;62(2):412–22.
Huo J, Dijokaite-Guraliuc A, Liu C, Zhou D, Ginn HM, Das R, Supasa P, Selvaraj M, Nutalai R, Tuekprakhon A. A delicate balance between antibody evasion and ACE2 affinity for Omicron BA. 2.75. Cell Rep. 2022;42:111903.
Cao Y, Song W, Wang L, Liu P, Yue C, Jian F, Yu Y, Yisimayi A, Wang P, Wang Y. Characterization of the enhanced infectivity and antibody evasion of Omicron BA. 2.75. Cell Host Microbe. 2022;30(11):1527–39.
Chakraborty C, Bhattacharya M, Sharma AR. Emerging mutations in the SARS-CoV-2 variants and their role in antibody escape to small molecule-based therapeutic resistance. Curr Opin Pharmacol. 2022;62:64–73.
Yue C, Song W, Wang L, Jian F, Chen X, Gao F, Shen Z, Wang Y, Wang X, Cao Y. Enhanced transmissibility of XBB. 1.5 is contributed by both strong ACE2 binding and antibody evasion. bioRxiv 2023:2023.2001. 2003.522427.
Pardo J, Shukla AM, Chamarthi G, Gupte A. The journey of remdesivir: from Ebola to COVID-19. Drugs Context. 2020;9:1–9.
Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID-19: a systematic review. Clin Epidemiol Glob Health. 2021;9:123–7.
Kumari M, Subbarao N. Deep learning model for virtual screening of novel 3C-like protease enzyme inhibitors against SARS coronavirus diseases. Comput Biol Med. 2021;132:104317.
Srinivasan S, Batra R, Chan H, Kamath G, Cherukara MJ, Sankaranarayanan SK. Artificial intelligence-guided De novo molecular design targeting COVID-19. ACS Omega. 2021;6(19):12557–66.
Bung N, Krishnan SR, Bulusu G, Roy A. De novo design of new chemical entities for SARS-CoV-2 using artificial intelligence. Future Med Chem. 2021;13(6):575–85.
Arshia AH, Shadravan S, Solhjoo A, Sakhteman A, Sami A. De novo design of novel protease inhibitor candidates in the treatment of SARS-CoV-2 using deep learning, docking, and molecular dynamic simulations. Comput Biol Med. 2021;139: 104967.
Magar R, Yadav P, Barati Farimani A. Potential neutralizing antibodies discovered for novel corona virus using machine learning. Sci Rep. 2021;11(1):1–11.
Williams AH, Zhan C-G. Fast prediction of binding affinities of SARS-CoV-2 spike protein and its mutants with antibodies through intermolecular interaction modeling-based machine learning. J Phys Chem B. 2022;126(28):5192–206.
Xu Z, Yang L, Zhang X, Zhang Q, Yang Z, Liu Y, Wei S, Liu W. Discovery of potential flavonoid inhibitors against COVID-19 3CL proteinase based on virtual screening strategy. Front Mol Biosci. 2020;7: 556481.
Li Z, Lin Y, Huang Y-Y, Liu R, Zhan C-G, Wang X, Luo H-B. Reply to Ma and Wang: Reliability of various in vitro activity assays on SARS-CoV-2 main protease inhibitors. Proc Natl Acad Sci USA. 2021;118(8):e2024937118. https://doi.org/10.1073/pnas.2024937118.
Ngwa W, Kumar R, Thompson D, Lyerly W, Moore R, Reid T-E, Lowe H, Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25(11):2707.
Pitsillou E, Liang J, Ververis K, Lim KW, Hung A, Karagiannis TC. Identification of small molecule inhibitors of the deubiquitinating activity of the SARS-CoV-2 papain-like protease: in silico molecular docking studies and in vitro enzymatic activity assay. Front Chem. 2020;8:623971.
Choi J, Yun JS, Song H, Kim NH, Kim HS, Yook JI. Exploring the chemical space of protein–protein interaction inhibitors through machine learning. Sci Rep. 2021;11(1):1–10.
Christensen AS, Faber FA, von Lilienfeld OA. Operators in quantum machine learning: response properties in chemical space. J Chem Phys. 2019;150(6):064105.
Coley CW. Defining and exploring chemical spaces. Trends Chem. 2021;3(2):133–45.
Deng Z-L, Du C-X, Li X, Hu B, Kuang Z-K, Wang R, Feng S-Y, Zhang H-Y, Kong D-X. Exploring the biologically relevant chemical space for drug discovery. J Chem Inf Model. 2013;53(11):2820–8.
Öztürk H, Özgür A, Schwaller P, Laino T, Ozkirimli E. Exploring chemical space using natural language processing methodologies for drug discovery. Drug Discov Today. 2020;25(4):689–705.
Ramakrishnan R, von Lilienfeld OA. Machine learning, quantum chemistry, and chemical space. Rev Comput Chem. 2017;30:225–56.
Reymond J-L. The chemical space project. Acc Chem Res. 2015;48(3):722–30.
Sperandio O, Reynès CH, Camproux A-C, Villoutreix BO. Rationalizing the chemical space of protein–protein interaction inhibitors. Drug Discov Today. 2010;15(5–6):220–9.
Kingma DP, Mohamed S, Jimenez Rezende D, Welling M. Semi-supervised learning with deep generative models. Adv Neural Inf Process Syst. 2014;27:1–16.
Harshvardhan G, Gourisaria MK, Pandey M, Rautaray SS. A comprehensive survey and analysis of generative models in machine learning. Comput Sci Rev. 2020;38: 100285.
Salakhutdinov R. Learning deep generative models. Annu Rev Stat Appl. 2015;2:361–85.
Davies M, Nowotka M, Papadatos G, Dedman N, Gaulton A, Atkinson F, Bellis L, Overington JP. ChEMBL web services: streamlining access to drug discovery data and utilities. Nucleic Acids Res. 2015;43(W1):W612–20.
Irwin JJ, Shoichet BK. ZINC–a free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45(1):177–82.
Sterling T, Irwin JJ. ZINC 15—ligand discovery for everyone. J Chem Inf Model. 2015;55(11):2324–37.
Santana MV, Silva-Jr FP. De novo design and bioactivity prediction of SARS-CoV-2 main protease inhibitors using recurrent neural network-based transfer learning. BMC Chem. 2021;15(1):8.
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25.
Liu G, Zeng H, Mueller J, Carter B, Wang Z, Schilz J, Horny G, Birnbaum ME, Ewert S, Gifford DK. Antibody complementarity determining region design using high-capacity machine learning. Bioinformatics. 2020;36(7):2126–33.
Akbar R, Robert PA, Weber CR, Widrich M, Frank R, Pavlović M, Scheffer L, Chernigovskaya M, Snapkov I, Slabodkin A. In silico proof of principle of machine learning-based antibody design at unconstrained scale. In: MAbs: 2022. Taylor & Francis. p. 2031482.
Morgan RS, McAdon JM. Predictor for sulfur-aromatic interactions in globular proteins. Int J Peptide Protein Res. 1980;15(2):177–80.
Williams AH, Zhan C-G. Generalized methodology for the quick prediction of variant SARS-CoV-2 spike protein binding affinities with human angiotensin-converting enzyme II. J Phys Chem B. 2022;126(12):2353–60.
Williams AH, Zhan C-G. Fast prediction of binding affinities of SARS-CoV-2 spike protein and its mutants with antibodies through intermolecular interaction modeling-based machine learning. J Phys Chem B. 2022;126(28):5194–206.
Williams AH, Zhan C-G. Fast prediction of binding affinities of the SARS-CoV-2 spike protein mutant N501Y (UK variant) with ACE2 and miniprotein drug candidates. J Phys Chem B. 2021;125(17):4330–6.
Guan D, Rahman MT, Gay EA, Vasukuttan V, Mathews KM, Decker AM, Williams AH, Zhan C-G, Jin C. Indole-containing amidinohydrazones as nonpeptide, dual RXFP3/4 agonists: synthesis, structure–activity relationship, and molecular modeling studies. J Med Chem. 2021;64(24):17866–86.
Yang J-F, Williams AH, Penthala NR, Prather PL, Crooks PA, Zhan C-G. Binding Modes and selectivity of cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptor ligands. ACS Chem Neurosci. 2020;11(20):3455–63.
Williams AH, Zhan C-G. Generalized methodology for the quick prediction of variant SARS-CoV-2 spike protein binding affinities with human angiotensin-converting enzyme II. J Phys Chem B. 2022;126(12):2353–60.
Srivastava HK, Sastry GN. Molecular dynamics investigation on a series of HIV protease inhibitors: assessing the performance of MM-PBSA and MM-GBSA approaches. J Chem Inf Model. 2012;52(11):3088–98.
Rastelli G, Del Rio A, Degliesposti G, Sgobba M. Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J Comput Chem. 2010;31(4):797–810.
Weng G, Wang E, Chen F, Sun H, Wang Z, Hou T. Assessing the performance of MM/PBSA and MM/GBSA methods. 9. Prediction reliability of binding affinities and binding poses for protein–peptide complexes. Phys Chem Chem Phys. 2019;21(19):10135–45.
Sun H, Duan L, Chen F, Liu H, Wang Z, Pan P, Zhu F, Zhang JZ, Hou T. Assessing the performance of MM/PBSA and MM/GBSA methods. 7. Entropy effects on the performance of end-point binding free energy calculation approaches. Phys Chem Chem Phys. 2018;20(21):14450–60.
Chen F, Sun H, Wang J, Zhu F, Liu H, Wang Z, Lei T, Li Y, Hou T. Assessing the performance of MM/PBSA and MM/GBSA methods. 8. Predicting binding free energies and poses of protein–RNA complexes. RNA. 2018;24(9):1183–94.
Chen F, Liu H, Sun H, Pan P, Li Y, Li D, Hou T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein–protein binding free energies and re-rank binding poses generated by protein–protein docking. Phys Chem Chem Phys. 2016;18(32):22129–39.
Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov. 2015;10(5):449–61.
Williams A, Zhou S, Zhan C-G. Discovery of potent and selective butyrylcholinesterase inhibitors through the use of pharmacophore-based screening. Bioorg Med Chem Lett. 2019;29(24): 126754.
Dong L, Qu X, Zhao Y, Wang B. Prediction of binding free energy of protein–ligand complexes with a hybrid molecular mechanics/generalized born surface area and machine learning method. ACS Omega. 2021;6(48):32938–47.
DeJong C, Wachter RM. The risks of prescribing hydroxychloroquine for treatment of COVID-19—first, do no harm. JAMA Intern Med. 2020;180(8):1118–9.
Chai PR, Ferro EG, Kirshenbaum JM, Hayes BD, Culbreth SE, Boyer EW, Erickson TB. Intentional hydroxychloroquine overdose treated with high-dose diazepam: an increasing concern in the COVID-19 pandemic. J Med Toxicol. 2020;16:314–20.
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell discovery. 2020;6(1):16.
Temple C, Hoang R, Hendrickson RG. Toxic effects from ivermectin use associated with prevention and treatment of Covid-19. N Engl J Med. 2021;385(23):2197–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Conceptualization, CG Zhan; Data curation and analysis, AH Williams; Writing – original draft preparation, AH Williams; Writing – review and editing, AH Williams and CG Zhan; Funding acquisition, CG Zhan. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the funding of the Molecular Modeling and Biopharmaceutical Center at the University of Kentucky College of Pharmacy and the National Science Foundation (Directorate for Mathematical and Physical Sciences, NSF Grant DMS-2245903 under funding opportunity NSF 22-600—Joint DMS/NIGMS Initiative to Support Research at the Interface of the Biological and Mathematical Sciences).
Conflicts of interest
Authors declare no conflicting interests concerning the information presented within this review.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and material
Not Applicable.
Code availability
Not Applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, A.H., Zhan, CG. Staying Ahead of the Game: How SARS-CoV-2 has Accelerated the Application of Machine Learning in Pandemic Management. BioDrugs 37, 649–674 (2023). https://doi.org/10.1007/s40259-023-00611-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-023-00611-8