Abstract
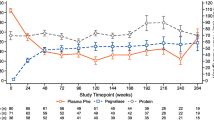
BioMarin Pharmaceutical is developing pegvaliase (PALYNZIQ™) as a treatment for phenylketonuria, a genetic disorder caused by deficiency of phenylalanine hydroxylase which leads to neurotoxic accumulation of phenylalanine. Data from the phase III PRISM clinical trial program indicate treatment with pegvaliase is associated with sustained reductions in blood phenylalanine levels and sustained improvements in neurological sequelae in patients with phenylketonuria. Based on these positive results pegvaliase was recently approved in the US for adults with phenylketonuria who have uncontrolled blood phenylalanine concentrations on current treatment. This article summarizes the milestones in the development of pegvaliase leading to this first approval.
Similar content being viewed by others
References
BioMarin Pharmaceutical Inc. Pegvaliase (Palynziq): US prescribing information; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761079s000lbl.pdf. Accessed 10 July 2018.
FDA. FDA approves a new treatment for PKU, a rare and serious genetic disease (media release); 2018. http://www.fda.gov.
Serono International. BioMarin and Serono form strategic alliance for the development and commercialization of phenoptin(TM) and phenylase(TM) (media release); 2005. http://www.serono.com.
BioMarin Pharmaceutical Inc. BioMarin to acquire rights to phenylketonuria (PKU) franchise from Merck Serono (media release); 2015. http://www.bmrn.com.
Longo N, Harding CO, Burton BK, et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open- label, multicentre, phase 1 dose-escalation trial. Lancet. 2014;384(No 9937):37–44.
Goldfinger M, Zeile WL, Corado CR, et al. Partial rescue of neuropathology in the murine model of PKU following administration of recombinant phenylalanine ammonia lyase (pegvaliase). Mol Genet Metab. 2017;122(1–2):33–5.
Thomas J, Levy H, Amato S, et al. Pegvaliase for the treatment of phenylketonuria: results of a long-term phase 3 clinical trial program (PRISM). Mol Genet Metab. 2018;124(1):27–38.
Zori R, Thomas JA, Shur N, et al. Evaluation of an induction, titration, and maintenance dosing regimen in a phase 2 study of ‘rAvPAL-PEG’ for control of blood phenylalanine levels in adults with phenylketonuria (PKU) (abstract no. P-150). J Inherit Metab Dis. 2015;38(Suppl 1):S117.
Longo N, Burton BK, Harding CO, et al. Hypophenylalaninemia in adult phenylketonuria patients treated with rAvPAL-PEG (abstract no. 67). Mol Genet Metab. 2012;105(3):335.
Longo N, Thomas J, Wasserstein M, et al. Evaluation of long-term safety and efficacy of pegvaliase treatment for adults with phenylketonuria: updated year 4 results (abstract no. O-013). J Inherit Metab Dis. 2016;39(Suppl 1):S39.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of Interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham, a contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Pegvaliase: First Global Approval. BioDrugs 32, 391–395 (2018). https://doi.org/10.1007/s40259-018-0292-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0292-3