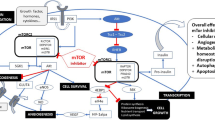
Abstract
The mammalian target of rapamycin inhibitor everolimus (Zortress®, Certican®) was recently approved in the USA and a number of EU countries for use in combination with a reduced dosage of tacrolimus and corticosteroids for the prophylaxis of organ rejection in adult liver transplant recipients. Compared with standard-exposure tacrolimus, early use of everolimus plus a reduced dosage of tacrolimus did not compromise efficacy in liver transplant recipients. In addition, significantly better renal function with everolimus plus reduced-exposure tacrolimus than with standard-exposure tacrolimus was seen from 6 weeks post-transplant onwards. Everolimus plus reduced-exposure tacrolimus has an acceptable tolerability profile in liver transplant recipients.


Similar content being viewed by others
References
Meier-Kriesche H-U, Li S, Gruessner RWG, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5 Pt 2):1111–31.
McGuire BM, Rosenthal P, Brown CC, et al. Long-term management of the liver transplant patient: recommendations for the primary care doctor. Am J Transplant. 2009;9(9):1988–2003.
Schuurman H-J, Cottens S, Fuchs S, et al. SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation. 1997;64(1):32–5.
Novartis. Zortress® (everolimus) tablets for oral use: US prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021560s006lbl.pdf. Accessed 8 Mar 2013.
Lakemedelsverket. Certican (everolimus) tablets: Swedish summary of product characteristics. 2012. http://www.lakemedelsverket.se/SPC_PIL/Pdf/enhumspc/Certican%20tablet%20ENG%20.doc. Accessed 8 Mar 2013.
Dunn C, Croom KF. Everolimus: a review of its use in renal and cardiac transplantation. Drugs. 2006;66(4):547–70.
Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64(1):36–42.
Schuurman H-J, Ringers J, Schuler W, et al. Oral efficacy of the macrolide immunosuppressant SDZ RAD and of cyclosporine microemulsion in cynomolgus monkey kidney allotransplantation. Transplantation. 2000;69(5):737–42.
Hausen B, Boeke K, Berry GJ, et al. Coadministration of Neoral and the novel rapamycin analog, SDZ RAD, to rat lung allograft recipients: potentiation of immunosuppressive efficacy and improvement of tolerability of staggered versus simultaneous treatment. Transplantation. 1999;67(7):956–62.
Hausen B, Ikonen T, Briffa N, et al. Combined immunosuppression with cyclosporine (Neoral) and SDZ RAD in non-human primate lung transplantation: systematic pharmacokinetic-based trials to improve efficacy and tolerability. Transplantation. 2000;69(1):76–86.
De Simone P, Metselaar HJ, Fischer L, et al. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transpl. 2009;15(10):1262–9.
Fischer L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation: PROTECT. Am J Transplant. 2012;12(7):1855–65.
Masetti M, Montalti R, Rompianesi G, et al. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10(10):2252–62.
De Simone P, Nevens F, De Carlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12(11):3008–20.
Disclosure
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: F. Saliba, Centre Hépato-Biliaire, Hôpital Paul Brousse, Villejif, France.
Rights and permissions
About this article
Cite this article
Keating, G.M., Lyseng-Williamson, K.A. Everolimus: A Guide to Its Use in Liver Transplantation. BioDrugs 27, 407–411 (2013). https://doi.org/10.1007/s40259-013-0041-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-013-0041-6