Abstract
Introduction
Due to the increase in healthcare budget constraint, economic evaluation (EE) evidence is increasingly required to inform resource allocation decisions. This study aimed to systematically review quantity, characteristics, and quality of full EE studies on diagnostic and therapeutic interventions conducted in 26 Middle East and North Africa (MENA) countries.
Methods
PubMed and Scopus databases were comprehensively searched to identify the published EE studies in the MENA region. The quality of reviewed studies was evaluated using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Results
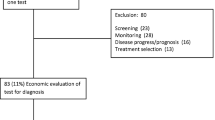
The search identified 69 studies. The cost-utility approach was adopted in 49 studies (71 %). More than half (38 studies; 55 %) were conducted in Iran and Turkey. Sixteen countries (62 %) did not have any EE studies. The most frequently analyzed therapeutic areas were infectious diseases (19 studies; 28 %), cardiovascular diseases (11 studies; 16 %), and malignancies (10 studies; 14 %). Ten studies (14 %), 46 (67 %), 12 (17 %), and 1 study (1 %) were classified as excellent, high, moderate, and poor quality, respectively. The mean of items reported was 85.10 % (standard deviation 13.32 %). Characterizing heterogeneity, measurement of effectiveness, time horizon, and discount rate were missed in 21 (60 %), 22 (32 %), 20 (29 %) and 15 (25 %) studies, respectively. Data on effectiveness and utility relied primarily on studies conducted outside the region.
Conclusions
The quantity of EE studies in the MENA region remains low; however, overall quality is high to excellent. The availability of local data, capacity building, and national guidelines are vital to improve both the quantity and quality of EE studies in the region.


Similar content being viewed by others
References
Abed GT, Davoodi HR. Challenges of growth and globalization in the Middle East and North Africa: International Monetary Fund; 2003.
Asbu EZ, Masri MD, Kaissi A. Health status and health systems financing in the MENA region: roadmap to universal health coverage. Glob Health Res Policy. 2017;2:25.
Fasseeh A, Karam R, Jameleddine M, George M, Kristensen FB, Al-Rabayah AA, et al. Implementation of health technology assessment in the Middle East and North Africa: Comparison between the current and preferred status. Front Pharmacol. 2020;11:15.
Thomas D, Hiligsmann M, John D, Al Ahdab OG, Li H. Chapter 18—pharmacoeconomic analyses and modeling. In: Thomas D, editor. Clinical pharmacy education, practice and research. New York: Elsevier; 2019. p. 261–75.
National Academies of Sciences E, Medicine. Making medicines affordable: a national imperative. New York: National Academies Press; 2018.
W. H. O. Using Health Technology Assessment for universal health coverage and reimbursement systems. Geneva, Switzerland: World Health Organization. 2016. https://www.who.int/health-technology-assessment/HTA_November_meeting_report_Final.pdf. Accessed 30 May 2021
Zrubka Z, Rashdan O, Gulácsi L. Health economic publications from the Middle East and North Africa Region: a scoping review of the volume and methods of research. Glob J Qual Saf Healthc. 2020;3(2):44–54.
Alefan Q, Rascati K. Pharmacoeconomic studies in World Health Organization Eastern mediterranean countries: reporting completeness. Int J Technol Assess Health Care. 2017;33(2):215–21.
Eljilany I, El-Dahiyat F, Curley LE, Babar ZU. Evaluating quantity and quality of literature focusing on health economics and pharmacoeconomics in Gulf Cooperation Council countries. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):403–14.
AlAujan SS, Almazrou SH, Al-Aqeel SA. A systematic review of sources of outcomes and cost data utilized in economic evaluation research conducted in the Gulf Cooperation Council. Risk Manag Healthc Policy. 2021;14:209–20.
Farid S, Elmahdawy M, Baines D. A systematic review on the extent and quality of pharmacoeconomic publications in Egypt. Clin Drug Investig. 2019;39(2):157–68.
Haghparast-Bidgoli H, Kiadaliri AA, Skordis-Worrall J. Do economic evaluation studies inform effective healthcare resource allocation in Iran? A critical review of the literature. Cost Effect Resour Alloc. 2014;12(1):15.
Al-Aqeel SA. State of health economic evaluation research in Saudi Arabia: a review. ClinicoEcon Outcomes Res. 2012;4:177–84.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–50.
Watts RD, Li IW. Use of checklists in reviews of health economic evaluations, 2010 to 2018. Value Health J Int Soc Pharmacoecon Outcomes Res. 2019;22(3):377–82.
Geng J, Yu H, Mao Y, Zhang P, Chen Y. Cost effectiveness of dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Pharmacoeconomics. 2015;33(6):581–97.
Aboutorabi A, Hadian M, Ghaderi H, Salehi M, Ghiasipour M. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2015;7(1):98–106.
Abushanab D, Alsoukhni O, AbouNahia F, Al-Badriyeh D. Clinical and economic analysis of morphine versus fentanyl in managing ventilated neonates with respiratory distress syndrome in the intensive care setting. Clin Ther. 2019;41(4):714-27.e8.
Alavian SM, Nikfar S, Kebriaeezadeh A, Lotfi F, Sanati E, RezaeiHemami M, et al. A cost-utility analysis of different antiviral medicine regimens in patients with chronic hepatitis C virus genotype 1 infection. Iran Red Crescent Med J. 2016;18(11):e37094-e.
Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. HMG versus rFSH for ovulation induction in developing countries: a cost-effectiveness analysis based on the results of a recent meta-analysis. Reprod Biomed Online. 2006;12(2):163–9.
Almalki Z, Alatawi Y, Alharbi A, Almaklefi B, Alfaiz S, Almohana O, et al. Cost-effectiveness of more intensive blood pressure treatment in patients with high risk of cardiovascular disease in saudi arabia: a modelling study of meta-analysis. Int J Hypertens. 2019;20:19.
Almalki ZS, Iqbal MS, Alablan FM, Alenazi RK, Tasha AR, Daghar MF, et al. Long term cost-effectiveness of a systolic blood pressure goal of <120 mmhg in hypertensive patients without diabetes mellitus. Value Health Reg Issues. 2020;21:157–63.
Alonso S, Tachfouti N, Najdi A, Sicuri E, Picado A. Cost-effectiveness of diagnostic-therapeutic strategies for paediatric visceral leishmaniasis in Morocco. BMJ Glob Health. 2017;2(3):e000315.
Alsaqa’aby MF, Vaidya V, Khreis N, Khairallah TA, Al-Jedai AH. Cost-effectiveness of oral agents in relapsing-remitting multiple sclerosis compared to interferon-based therapy in Saudi Arabia. Ann Saudi Med. 2017;37(6):433–43.
Amiri A, Goudarzi R, Amiresmaili M, Iranmanesh F. Cost-effectiveness analysis of tissue plasminogen activator in acute ischemic stroke in Iran. J Med Econ. 2018;21(3):282–7.
Amirsadri M, Hassani A. Cost-effectiveness and cost-utility analysis of OTC use of simvastatin 10 mg for the primary prevention of myocardial infarction in Iranian men. Daru J Faculty Pharm Tehran Univ Med Sci. 2015;30(23):56.
Amirsadri M, Mousavi S, Karimipour A. The cost-effectiveness and cost-utility analysis of the use of enoxaparin compared with heparin for venous thromboembolism prophylaxis in medical inpatients in Iran. Daru J Faculty Pharm Tehran Univ Med Sci. 2019;27(2):627–34.
Ansaripour A, Uyl-de Groot CA, Redekop WK. Adjuvant trastuzumab therapy for early HER2-positive breast cancer in Iran: a cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics. 2018;36(1):91–103.
Ashoush N. Economic evaluation of imipenem-cilastatin versus doripenem in ventilator-associated pneumonia in Egypt. Asian J Pharm Clin Res. 2017;10(6):406–10.
Bayazidi Y, Keshtkaran A, Homaie Rad E, Ansari M, Javanbakht M, Hashemi Meshkini A, et al. Cost-utility analysis of single-fraction versus multiple-fraction radiotherapy in patients with painful bone metastases: an Iranian patient’s perspective study. Value Health Reg Issues. 2017;12:84–9.
Cardarelli M, Vaikunth S, Mills K, DiSessa T, Molloy F, Sauter E, et al. Cost-effectiveness of humanitarian pediatric cardiac surgery programs in low- and middle-income countries. JAMA Netw Open. 2018;1(7):e184707.
Davari M, Ashrafi F, Maracy M, Aslani A, Tabatabaei M. Cost-effectiveness analysis of cetuximab in treatment of metastatic colorectal cancer in Iranian pharmaceutical market. Int J Prev Med. 2015;20:15.
Davies A, Bakhai A, Schmitt C, Barrett A, Graham-Clarke P, Sculpher M. Prasugrel vs clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a model-based cost-effectiveness analysis for Germany, Sweden, the Netherlands, and Turkey. J Med Econ. 2013;16(4):510–21.
Ebadi Fard Azar AA, Rezapour A, Alipour V, Sarabi-Asiabar A, Gray S, Mobinizadeh M, et al. Cost-effectiveness of teriparatide compared with alendronate and risedronate for the treatment of postmenopausal osteoporosis patients in Iran. Med J Islam Repub Iran. 2017;31:39.
El-Dahiyat F, Eljilany I. Cost-minimization analysis of ranibizumab versus aflibercept for treating Saudi patients with visual impairment owing to age-related macular degeneration or diabetic macular edema. Value Health Reg Issues. 2020;22:23–6.
Elsisi GH, Aburawash A, Waked E. Cost-effectiveness analysis of new hepatitis C virus treatments in Egyptian cirrhotic and noncirrhotic patients: a societal perspective. Value Health Reg Issues. 2017;13:7–15.
Elsisi GH, Nada Y, Rashad N, Carapinha J, Noor AO, Almasri DM, et al. Cost-effectiveness of six months versus 1-year adjuvant trastuzumab in HER2 positive early breast cancer in Egypt. J Med Econ. 2020;23(6):575–80.
Elsisi GH, Nada Y, Rashad N, Carapinha J. Cost-effectiveness of sorafenib versus best supportive care in advanced hepatocellular carcinoma in Egypt. J Med Econ. 2019;22(2):163–8.
Elsisi GH, Eldessouki R, Kalo Z, Elmazar MM, Taha AS, Awad BF, et al. Cost-effectiveness of the combined use of warfarin and low-dose aspirin versus warfarin alone in Egyptian patients with aortic valve replacements: a Markov model. Value Health Reg Issues. 2014;4(1):24–30.
Emmett SD, Sudoko CK, Tucci DL, Gong W, Saunders JE, Akhtar N, et al. Expanding access: cost-effectiveness of cochlear implantation and deaf education in Asia. Otolaryngol Head Neck Surg. 2019;161(4):672–82.
Ginsberg GM, Chemtob D. Cost utility analysis of HIV pre exposure prophylaxis among men who have sex with men in Israel. BMC Public Health. 2020;20(1):271.
Goldstein DA, Chen Q, Ayer T, Chan KKW, Virik K, Hammerman A, et al. Bevacizumab for metastatic colorectal cancer: a global cost-effectiveness analysis. Oncologist. 2017;22(6):694–9.
Golestani M, Eshghi P, Rasekh HR, Cheraghali AM, Salamzadeh J, Naderi M, et al. Cost-effectiveness analysis of biogeneric recombinant activated factor VII (AryoSeven™) and activated prothrombin complex concentrates (FEIBA™) to treat hemophilia a patients with inhibitors in Iran. Iran J Pharm Res. 2016;15(2):669–77.
Gupta V, Baabbad R, Hammerby E, Nikolajsen A, Shafie AA. An analysis of the cost-effectiveness of switching from biphasic human insulin 30, insulin glargine, or neutral protamine Hagedorn to biphasic insulin aspart 30 in people with type 2 diabetes. J Med Econ. 2015;18(4):263–72.
Hashemi Meshkini A, Keshavarz K, Gharibnaseri Z, Nikfar S, Abdollahi M. The effectiveness and cost-effectiveness of pregabalin in the treatment of diabetic peripheral neuropathy: a systematic review and economic model. Int J Pharmacol. 2012;8:496–509.
Hashemi-Meshkini A, Nikfar S, Glaser E, Jamshidi A, Hosseini SA. Cost-effectiveness analysis of tocilizumab in comparison with infliximab in Iranian rheumatoid arthritis patients with inadequate response to tDMARDs: a multistage markov model. Value Health Reg Issues. 2016;9:42–8.
Hersi AS, Osenenko KM, Kherraf SA, Aziz AA, Sambrook RJ. Cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation in Saudi Arabia. Ann Saudi Med. 2019;39(4):265–78.
Home P, Baik SH, Gálvez GG, Malek R, Nikolajsen A. An analysis of the cost-effectiveness of starting insulin detemir in insulin-naïve people with type 2 diabetes. J Med Econ. 2015;18(3):230–40.
Jabbari A, Jafari A, Hadian M, Ghasemi M. Model-based cost-effectiveness analysis of atorvastatin drugs for prevention of cardiovascular diseases in Iran. Int J Prev Med. 2020;11:57.
Kavosi Z, Sarikhani Khorrami M, Keshavarz K, Jafari A, Hashemi Meshkini A, Safaei HR, et al. Is Taurolidine-citrate an effective and cost-effective hemodialysis catheter lock solution? A systematic review and cost-effectiveness analysis. Med J Islam Repub Iran. 2016;30:347.
Keshavarz K, Kebriaeezadeh A, Alavian SM, Sari AA, Hemami MR, Lotfi F, et al. A cost-utility and cost-effectiveness analysis of different oral antiviral medications in patients with HBeAg-negative chronic hepatitis B in Iran: an economic microsimulation decision model. Hepat Mon. 2016;16:9.
Keshtkaran A, Javanbakht M, Salavati S, Mashayekhi A, Karimi M, Nuri B. Cost-utility analysis of oral deferasirox versus infusional deferoxamine in transfusion-dependent β-thalassemia patients. Transfusion. 2013;53(8):1722–9.
Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health J Int Soc Pharmacoecon Outcomes Res. 2010;13(4):381–7.
Koçkaya G, Yenilmez FB, Ergin G, Atikeler K, Tatar M. Cost effectiveness and economic value of obesity surgery for Turkey (CEVOS-T). Obes Med. 2016;1:33–7.
Kockaya G, Kose A, Yenilmez FB, Ozdemir O, Kucuksayrac E. Cost-effectiveness analysis of oral anti-viral drugs used for treatment of chronic hepatitis B in Turkey. Cost Eff Resour Allocat. 2015;13:1.
Li B, Miners A, Shakur H, Roberts I. Tranexamic acid for treatment of women with post-partum haemorrhage in Nigeria and Pakistan: a cost-effectiveness analysis of data from the WOMAN trial. Lancet Glob Health. 2018;6(2):e222–8.
Lichtenberg FR, Tatar M, Çalişkan Z. The effect of pharmaceutical innovation on longevity, hospitalization and medical expenditure in Turkey, 1999–2010. Health Policy. 2014;117(3):361–73.
Lim AG, Walker JG, Mafirakureva N, Khalid GG, Qureshi H, Mahmood H, et al. Effects and cost of different strategies to eliminate hepatitis C virus transmission in Pakistan: a modelling analysis. Lancet Glob Health. 2020;8(3):e440–50.
Mehrazmay A, Alavian SM, Moradi-Lakeh M, Payam MM, Hashemi-Meshkini A, Behnava B, et al. Cost-effectiveness analysis of adding low dose ribavirin to peginterferon alfa-2a for treatment of chronic hepatitis C infected thalassemia major patients in Iran. Hepat Mon. 2013;13:9.
Mokhtari-Payam M, Moradi-Lakeh M, Yaghoubi M, Moradijou M. Cost-effectiveness analysis of confocal scan laser ophthalmoscope (HRT II) versus GDX for diagnosing glaucoma. J Curr Ophthalmol. 2015;27(1–2):16–20.
Nasef SA, Shaaban AA, Mould-Quevedo J, Ismail TA. The cost-effectiveness of celecoxib versus non-steroidal anti-inflammatory drugs plus proton-pump inhibitors in the treatment of osteoarthritis in Saudi Arabia. Health Econ Rev. 2015;5:1.
Nasser SC, Mansour H, Abi Nader T, Metni M. Cost-effectiveness of novel treatment of hepatitis C virus in Lebanese patients. Int J Clin Pharm. 2018;40(3):693–9.
Neoh CF, Senol E, Kara A, Dinleyici EC, Turner SJ, Kong DCM. Cost-effectiveness analysis of anidulafungin vs fluconazole for the treatment of invasive candidiasis (IC) in Turkey. Mycoses. 2017;60(11):714–22.
Neoh CF, Senol E, Kara A, Dinleyici EC, Turner SJ, Kong DCM. Economic evaluation of micafungin versus liposomal amphotericin B (LAmB) for treating patients with candidaemia and invasive candidiasis (IC) in Turkey. Eur J Clin Microbial Infect Dis. 2018;37(9):1777–84.
Neoh CF, Senol E, Kara A, Dinleyici EC, Turner SJ, Kong DCM. Pharmacoeconomic evaluation of micafungin versus caspofungin as definitive therapy for candidaemia and invasive candidiasis (IC) in Turkey. Eur J Clin Microbial Infect Dis. 2018;37(3):537–44.
Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. Daru J Faculty Pharm Tehran Univ Med Sci. 2013;21(1):50.
Nuhoho S, Saad A, Saumell G, Ribes D, El Khoury AC. Economic evaluation of paliperidone palmitate once monthly for treating chronic schizophrenia patients in the United Arab Emirates. Curr Med Res Opin. 2018;34(4):601–11.
Obach D, Deuffic-Burban S, Esmat G, Anwar WA, Dewedar S, Canva V, et al. Effectiveness and cost-effectiveness of immediate versus delayed treatment of hepatitis C virus-infected patients in a country with limited resources: the case of Egypt. Clin Infect Dis. 2014;58(8):1064–71.
Rasekh HR, Imani A, Karimi M, Golestani M. Cost-utility analysis of immune tolerance induction therapy versus on-demand treatment with recombinant factor VII for hemophilia A with high titer inhibitors in Iran. Clin Outcomes Res. 2011;3(1):207–12.
Rizk R, Hiligsmann M, Karavetian M, Evers S. Cost-effectiveness of dedicated dietitians for hyperphosphatemia management among hemodialysis patients in Lebanon: results from the Nutrition Education for Management of Osteodystrophy trial. J Med Econ. 2017;20(10):1024–38.
Roze S, Smith-Palmer J, de Portu S, Özdemir Saltik AZ, Akgül T, Deyneli O. Cost-effectiveness of sensor-augmented insulin pump therapy versus continuous insulin infusion in patients with type 1 diabetes in Turkey. Diabetes Technol Ther. 2019;21(12):727–35.
Saiyarsarai P, Khorasani E, Photogeraphy H, Ghaffari Darab M, Seyedifar M. Cost-utility of new film-coated tablet formulation of deferasirox vs deferoxamine among major beta-thalassemia patients in Iran. Medicine (Baltimore). 2020;99(28):e20949-e.
Sari AA, Ravaghi H, Mobinizadeh M, Sarvari S. The cost-utility analysis of PET-scan in diagnosis and treatment of non-small cell lung carcinoma in Iran. Iran J Radiol. 2013;10:2.
Saylan M, Treur MJ, Postema R, Dilbaz N, Savas H, Heeg BM, et al. Cost-effectiveness analysis of aripiprazole augmentation treatment of patients with major depressive disorder compared to olanzapine and quetiapine augmentation in Turkey: A microsimulation approach. Value Health Reg Issues. 2013;2(2):171–80.
Shafie AA, Gupta V, Baabbad R, Hammerby E, Home P. An analysis of the short- and long-term cost-effectiveness of starting biphasic insulin aspart 30 in insulin-naïve people with poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2014;106(2):319–27.
Sutherland T, Downing J, Miller S, Bishai DM, Butrick E, Fathalla MM, et al. Use of the non-pneumatic anti-shock garment (NASG) for life-threatening obstetric hemorrhage: a cost-effectiveness analysis in Egypt and Nigeria. PLoS One. 2013;8(4):e62282.
Taheri S, Sahraian MA, Yousefi N. Cost-effectiveness of alemtuzumab and natalizumab for relapsing-remitting multiple sclerosis treatment in Iran: decision analysis based on an indirect comparison. J Med Econ. 2019;22(1):71–84.
Taheri S, Heidari E, Aivazi MA, Shams-Beyranvand M, Varmaghani M. Cost-effectiveness analysis of Ivabradine in treatment of patients with heart failure in Iran. Int J Technol Assess Health Care. 2018;34(6):576–83.
Taheri S, Fashami FM, Peiravian F, Yousefi N. Teriparatide in the treatment of severe postmenopausal osteoporosis: A cost-utility analysis. Iran J Pharm Res. 2019;18(2):1073–85.
Toy M, Onder FO, Idilman R, Kabacam G, Richardus JH, Bozdayi M, et al. The cost-effectiveness of treating chronic hepatitis B patients in a median endemic and middle income country. Eur J Health Econ Health Econ Prev Care. 2012;13(5):663–76.
Turner SJ, Senol E, Kara A, Al-Badriyeh D, Dinleyici EC, Kong DC. Cost effectiveness of caspofungin vs voriconazole for empiric therapy in Turkey. Mycoses. 2014;57(8):489–96.
Turner SJ, Senol E, Kara A, Al-Badriyeh D, Kong DC, Dinleyici EC. Pharmacoeconomic evaluation of caspofungin versus liposomal amphotericin B in empirical treatment of invasive fungal infections in Turkey. Int J Antimicrob Agents. 2013;42(3):276–80.
Turner SJ, Senol E, Kara A, Al-Badriyeh D, Dinleyici EC, Kong DC. Pharmacoeconomic evaluation of voriconazole vs liposomal amphotericin B in empiric treatment of invasive fungal infections in Turkey. BMC Infect Dis. 2013;13:560.
Vassall A, Bagdadi S, Bashour H, Zaher H, Maaren PV. Cost-effectiveness of different treatment strategies for tuberculosis in Egypt and Syria. Int J Tuberculosis Lung Dis. 2002;6(12):1083–90.
Yalçin Balçik P, Şahin B. Cost-effectiveness analysis of pemetrexed and gemcitabine treatment for advanced nonsmall cell lung cancer in Turkey. Turk J Med Sci. 2016;46(1):152–8.
Tantivess S, Chalkidou K, Tritasavit N, Teerawattananon Y. Health Technology Assessment capacity development in low- and middle-income countries: Experiences from the international units of HITAP and NICE. Research. 2017;6:2119.
Decimoni T, Leandro R, Rozman L, Craig D, Iglesias C, Dutilh N, et al. Systematic review of health economic evaluation studies developed in Brazil from 1980 to 2013. Front Public Health. 2018;6:52.
Addo R, Goodall S, Hall J, Haas M. Assessing the capacity of Ghana to introduce health technology assessment: a systematic review of economic evaluations conducted in Ghana. Int J Technol Assess Health Care. 2020;36(5):500–7.
Luz A, Santatiwongchai B, Pattanaphesaj J, Teerawattananon Y. Identifying priority technical and context-specific issues in improving the conduct, reporting and use of health economic evaluation in low- and middle-income countries. Health Res Policy Syst. 2018;16(1):4.
Almazrou SH, Alaujan SS, Al-Aqeel SA. Barriers and facilitators to conducting economic evaluation studies of Gulf Cooperation Council (GCC) countries: a survey of researchers. Health Res Policy Syst. 2021;19(1):71.
Sharkawy MN, Dastan I. A scoping review of health economic evaluation in the World Health Organization Eastern Mediterranean region. Expert Rev Pharmacoecon Outcomes Res. 2021;20:20.
Adeagbo CU, Rattanavipapong W, Guinness L, Teerawattananon Y. The development of the guide to economic analysis and research (GEAR) online resource for low- and middle-income countries’ health economics practitioners: a commentary. Value Health J Int Soc Pharmacoecon Outcomes Res. 2018;21(5):569–72.
Kim T, Sharma M, Teerawattananon Y, Oh C, Ong L, Hangoma P, et al. Addressing challenges in health technology assessment institutionalization for furtherance of universal health coverage through south-south knowledge exchange: lessons from Bhutan, Kenya, Thailand, and Zambia. Value Health Reg Issues. 2021;24:187–92.
Almomani E, Alabbadi I, Fasseeh A, Al-Qutob R, Al-Sharu E, Hayek N, et al. Implementation road map of health technology assessment in middle-income countries: the case of Jordan. Value Health Regional Issues. 2021;25:126–34.
Al-Aqeel S. Health technology assessment in Saudi Arabia. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):393–402.
Chaikledkaew U, Kittrongsiri K. Guidelines for health technology assessment in Thailand (second edition)–the development process. J Med Assoc Thailand Chotmaihet Thangphaet. 2014;97(Suppl 5):S4-9.
Fikri M, Hammerich A. Scaling up action on the prevention and control of noncommunicable diseases in the WHO Eastern Mediterranean Region. East Mediterr Health J. 2018;24(1):3–4.
Tehrani-Banihashemi A, Moradi-Lakeh M, El Bcheraoui C, Charara R, Khalil I, Afshin A, et al. Burden of cardiovascular diseases in the Eastern Mediterranean Region, 1990–2015: findings from the Global Burden of Disease 2015 study. Int J Public Health. 2018;63(1):137–49.
Gagnon-Arpin I, Habib M, AlAyoubi F, Sutherland G, Dobrescu A, Villa G, et al. Modelling the burden of cardiovascular disease in Saudi Arabia and the impact of reducing modifiable risk factors. J Saudi Heart Assoc. 2018;30(4):365.
Asbu EZ, Masri MD, Kaissi AA. Health status and health systems financing in the MENA region: roadmap to universal health coverage. Glob Health Res Policy. 2017;2:20.
Leech AA, Kim DD, Cohen JT, Neumann PJ. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: trends in cost-per-daly studies. Value Health. 2018;21(7):759–61.
Woods B, Revill P, Sculpher M, Claxton K. Country-Level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health J Int Soc Pharmacoecon Outcomes Res. 2016;19(8):929–35.
Sharkawy MN, Dastan I. A scoping review of health economic evaluation in the World Health Organization Eastern Mediterranean region. Expert Rev Pharmacoecon Outcomes Res. 2021;22:20.
Nagi MA, Luangsinsiri C, Thavorncharoensap M (2021) A systematic review of economic evaluations of vaccines in Middle East and North Africa countries: is existing evidence good enough to support policy decision-making? Expert Rev Pharmacoecono Outcomes Res 21(6):1159–1178. https://doi.org/10.1080/14737167.2021.1954508
Acknowledgements
This work was part of the training of Mouaddh Abdulmalik Nagi at Social, Economic and Administrative pharmacy graduate program at Mahidol University. The authors would like to gratefully acknowledge Health Technology Assessment international (HTAi), Alberta—Canada and Mahidol University for their generous support of this training. The findings, interpretations and conclusions expressed in this article do not necessarily reflect the views of the aforementioned supporting agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for this research.
Conflict of Interest
Mouaddh Abdulmalik Nagi, Pramitha Esha Nirmala Dewi, Montarat Thavorncharoensap, and Sermsiri Sangroongruangsri declare that they have no conflict of interest.
Ethical Approval
Ethical approval is not required for this study since it does not include human subjects.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Author Contribution
All authors attest they meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MAN, MT, SS and PEND. The first draft of the manuscript was written by MAN. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagi, M.A., Dewi, P.E.N., Thavorncharoensap, M. et al. A Systematic Review on Economic Evaluation Studies of Diagnostic and Therapeutic Interventions in the Middle East and North Africa. Appl Health Econ Health Policy 20, 315–335 (2022). https://doi.org/10.1007/s40258-021-00703-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-021-00703-y