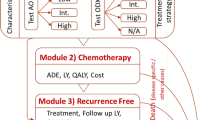
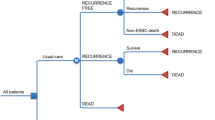
Abstract
Background
Tamoxifen therapy reduces the risk of breast cancer but increases the risk of serious adverse events including endometrial cancer and thromboembolic events.
Objectives
The cost effectiveness of using a commercially available breast cancer risk assessment test (BREVAGen™) to inform the decision of which women should undergo chemoprevention by tamoxifen was modeled in a simulated population of women who had undergone biopsies but had no diagnosis of cancer.
Methods
A continuous time, discrete event, mathematical model was used to simulate a population of white women aged 40–69 years, who were at elevated risk for breast cancer because of a history of benign breast biopsy. Women were assessed for clinical risk of breast cancer using the Gail model and for genetic risk using a panel of seven common single nucleotide polymorphisms. We evaluated the cost effectiveness of using genetic risk together with clinical risk, instead of clinical risk alone, to determine eligibility for 5 years of tamoxifen therapy. In addition to breast cancer, the simulation included health states of endometrial cancer, pulmonary embolism, deep-vein thrombosis, stroke, and cataract. Estimates of costs in 2012 US dollars were based on Medicare reimbursement rates reported in the literature and utilities for modeled health states were calculated as an average of utilities reported in the literature. A 50-year time horizon was used to observe lifetime effects including survival benefits.
Results
For those women at intermediate risk of developing breast cancer (1.2–1.66 % 5-year risk), the incremental cost-effectiveness ratio for the combined genetic and clinical risk assessment strategy over the clinical risk assessment-only strategy was US$47,000, US$44,000, and US$65,000 per quality-adjusted life-year gained, for women aged 40–49, 50–59, and 60–69 years, respectively (assuming a price of US$945 for genetic testing). Results were sensitive to assumptions about patient adherence, utility of life while taking tamoxifen, and cost of genetic testing.
Conclusions
From the US payer’s perspective, the combined genetic and clinical risk assessment strategy may be a moderately cost-effective alternative to using clinical risk alone to guide chemoprevention recommendations for women at intermediate risk of developing breast cancer.



Similar content being viewed by others
References
Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24.
Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88.
Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101.
Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet. 1998;352:93–7.
Chemoprevention of Breast Cancer. Recommendation and Rationale. Rockville, MD: Agency for Healthcare Research and Quality; 2002. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/3rduspstf/breastchemo/breastchemorr.htm. Accessed 28 May 2013.
Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:604–14.
Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58.
Armstrong K, Quistberg DA, Micco E, Domchek S, Guerra C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch Intern Med. 2006;166:2260–5.
Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86.
Breast Cancer Risk Assessment Tool (BCRAT). National Cancer Institute. http://www.cancer.gov/bcrisktool/. Accessed 20 Dec 2012.
Melnikow J, Kuenneth C, Helms LJ, et al. Chemoprevention: drug pricing and mortality: the case of tamoxifen. Cancer. 2006;107:950–8.
Noah-Vanhoucke J, Green LE, Dinh TA, Alperin P, Smith RA. Cost-effectiveness of chemoprevention of breast cancer using tamoxifen in a postmenopausal US population. Cancer. 2011;117:3322–31.
Clinical Practice Guidelines for Oncology. Breast Cancer Risk Reduction. Version 1.2013. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed 28 May 2013.
Mealiffe ME, Stokowski RP, Rhees BK, Prentice RL, Pettinger M, Hinds DA. Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst. 2010;102:1618–27.
Comen E, Balistreri L, Gonen M, et al. Discriminatory accuracy and potential clinical utility of genomic profiling for breast cancer risk in BRCA-negative women. Breast Cancer Res Treat. 2011;127:479–87.
Beery TA, Williams JK. Risk reduction and health promotion behaviors following genetic testing for adult-onset disorders. Genet Test. 2007;11:111–23.
Garcia-Closas M, Chanock S. Genetic susceptibility loci for breast cancer by estrogen receptor status. Clin Cancer Res. 2008;14:8000–9.
Garcia-Closas M, Hall P, Nevanlinna H, et al. Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008;4:e1000054.
Stacey SN, Manolescu A, Sulem P, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39:865–9.
Stacey SN, Manolescu A, Sulem P, et al. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2008;40:703–6.
Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–93.
National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. SEER*Stat Database. Surveillance, Epidemiology, and End Results (SEER) Program 2007; Incidence—SEER 13 Regs Limited-Use, Nov 2006 Sub (1992-2004).
Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169:1001–8.
Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–501.
Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300.
Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer: 96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82.
Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90.
Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–9.
National Health and Nutrition Examination Survey Data 1999-2006. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). http://www.cdc.gov.ezproxy.dominican.edu/nchs/nhanes.htm. Accessed 20 Dec 2012.
Pinsky PF, Kramer BS, Reding D, Buys S, PLCO Project Team. Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2003;157:792–9.
Consumer Price Index. Bureau of Labor Statistics, U.S. Department of Labor. http://www.bls.gov/cpi. Accessed 20 Dec 2012.
HealthWarehouse.com. (http://www.healthwarehouse.com. Accessed 20 Dec 2012.
Center for the Evaluation of Value and Risk in Health (CEA Registry). Tufts Medical Center Institute for Clinical Research and Health Policy Studies. https://research.tufts-nemc.org/cear4/default.aspx. Accessed 5 July 2011.
Mourits MJ, De Vries EG, Willemse PH, Ten Hoor KA, Hollema H, Van der Zee AG. Tamoxifen treatment and gynecologic side effects: a review. Obstet Gynecol. 2001;97:855–66.
Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–46.
Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526–32.
Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–49.
Ahmed S, Thomas G, Ghoussaini M, et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet. 2009;41:585–90.
Thomas G, Jacobs KB, Kraft P, et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet. 2009;41:579–84.
Gail MH. Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst. 2009;101:959–63.
Wacholder S, Hartge P, Prentice R, et al. Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010;362:986–93.
Melnikow J, Birch S, Slee C, McCarthy TJ, Helms LJ, Kuppermann M. Tamoxifen for breast cancer risk reduction: impact of alternative approaches to quality-of-life adjustment on cost-effectiveness analysis. Med Care. 2008;46:946–53.
Current Trials Working Party of the Cancer Research, Campaign Breast Cancer Trials Group. Preliminary results from the cancer research campaign trial evaluating tamoxifen duration in women aged fifty years or older with breast cancer. J Natl Cancer Inst. 1996;88:1834–9.
Spiegelman D, Colditz GA, Hunter D, Hertzmark E. Validation of the Gail et al. model for predicting individual breast cancer risk. J Natl Cancer Inst. 1994;86:600–7.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–59.
Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16.
Green LE, Dinh TA, Smith RA. An estrogen model: the relationship between body mass index, menopausal status, estrogen replacement therapy, and breast cancer risk. Comput Math Methods Med. 2012;2012:792375.
Hoover DR, Crystal S, Kumar R, Sambamoorthi U, Cantor JC. Medical expenditures during the last year of life: findings from the 1992–1996 Medicare current beneficiary survey. Health Serv Res. 2002;37:1625–42.
Auerbach AD, Sanders GD, Hambleton J. Cost-effectiveness of testing for hypercoagulability and effects on treatment strategies in patients with deep vein thrombosis. Am J Med. 2004;116:816–28.
Caro JJ, Getsios D, Caro I, O’Brien JA. Cost effectiveness of tinzaparin sodium versus unfractionated heparin in the treatment of proximal deep vein thrombosis. Pharmacoeconomics. 2002;20:593–602.
Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg. 2004;124:507–17.
Lekander I, Borgstrom F, Strom O, Zethraeus N, Kanis JA. Cost-effectiveness of hormone therapy in the United States. J Womens Health (Larchmt). 2009;18:1669–77.
Locker GY, Mansel R, Cella D, et al. Cost-effectiveness analysis of anastrozole versus tamoxifen as primary adjuvant therapy for postmenopausal women with early breast cancer: a US healthcare system perspective. The 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen Alone or in Combination) trial. Breast Cancer Res Treat. 2007;106:229–38.
Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G. Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med. 2001;111:130–9.
Chau Q, Cantor SB, Caramel E, et al. Cost-effectiveness of the bird’s nest filter for preventing pulmonary embolism among patients with malignant brain tumors and deep venous thrombosis of the lower extremities. Support Care Cancer. 2003;11:795–9.
Cykert S, Phifer N, Hansen C. Tamoxifen for breast cancer prevention: a framework for clinical decisions. Obstet Gynecol. 2004;104:433–42.
Mansel R, Locker G, Fallowfield L, Benedict A, Jones D. Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen alone or in combination) trial. Br J Cancer. 2007;97:152–61.
Salpeter SR, Buckley NS, Liu H, Salpeter EE. The cost-effectiveness of hormone therapy in younger and older postmenopausal women. Am J Med. 2009;122(42–52):e2.
ten Cate-Hoek AJ, Dielis AW, Spronk HM, et al. Thrombin generation in patients after acute deep-vein thrombosis. Thromb Haemost. 2008;100:240–5.
Brandle M, Azoulay M, Greiner RA. Cost-effectiveness and cost-utility of insulin glargine compared with NPH insulin based on a 10-year simulation of long-term complications with the Diabetes Mellitus Model in patients with type 2 diabetes in Switzerland. Int J Clin Pharmacol Ther. 2007;45:203–20.
Brown GC, Brown MM, Brown HC, Kindermann S, Sharma S. A value-based medicine comparison of interventions for subfoveal neovascular macular degeneration. Ophthalmology. 2007;114:1170–8.
Cohen N, Minshall ME, Sharon-Nash L, Zakrzewska K, Valentine WJ, Palmer AJ. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia. Pharmacoeconomics. 2007;25:881–97.
Grann VR, Sundararajan V, Jacobson JS, et al. Decision analysis of tamoxifen for the prevention of invasive breast cancer. Cancer J. 2000;6:169–78.
Hershman D, Sundararajan V, Jacobson JS, Heitjan DF, Neugut AI, Grann VR. Outcomes of tamoxifen chemoprevention for breast cancer in very high-risk women: a cost-effectiveness analysis. J Clin Oncol. 2002;20:9–16.
Hopkins RB, Tarride JE, Bowen J, et al. Cost-effectiveness of reducing wait times for cataract surgery in Ontario. Can J Ophthalmol. 2008;43:213–7.
Ruof J, Golay A, Berne C, Collin C, Lentz J, Maetzel A. Orlistat in responding obese type 2 diabetic patients: meta-analysis findings and cost-effectiveness as rationales for reimbursement in Sweden and Switzerland. Int J Obes (Lond). 2005;29:517–23.
Tunis SL, Minshall ME, Charles M, Pandya BJ, Baran RW. Pioglitazone versus rosiglitazone treatment in patients with type 2 diabetes and dyslipidemia: cost-effectiveness in the US. Curr Med Res Opin. 2008;24:3085–96.
Armstrong K, Chen TM, Albert D, Randall TC, Schwartz JS. Cost-effectiveness of raloxifene and hormone replacement therapy in postmenopausal women: impact of breast cancer risk. Obstet Gynecol. 2001;98:996–1003.
Delea TE, El-Ouagari K, Karnon J, Sofrygin O. Cost-effectiveness of letrozole versus tamoxifen as initial adjuvant therapy in postmenopausal women with hormone-receptor positive early breast cancer from a Canadian perspective. Breast Cancer Res Treat. 2008;108:375–87.
Sonnenberg FA, Burkman RT, Hagerty CG, Speroff L, Speroff T. Costs and net health effects of contraceptive methods. Contraception. 2004;69:447–59.
El Ouagari K, Karnon J, Delea T, Talbot W, Brandman J. Cost-effectiveness of letrozole in the extended adjuvant treatment of women with early breast cancer. Breast Cancer Res Treat. 2007;101:37–49.
Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol. 2004;22:854–63.
Kanis JA, Borgstrom F, Johnell O, Oden A, Sykes D, Jonsson B. Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporos Int. 2005;16:15–25.
Lee JH, Glick HA, Hayman JA, Solin LJ. Decision-analytic model and cost-effectiveness evaluation of postmastectomy radiation therapy in high-risk premenopausal breast cancer patients. J Clin Oncol. 2002;20:2713–25.
Lievens Y, Kesteloot K, van den Bogaert W. Economic consequence of local control with radiotherapy: cost analysis of internal mammary and medial supraclavicular lymph node radiotherapy in breast cancer. Int J Radiat Oncol Biol Phys. 2005;63:1122–31.
Norum J, Olsen JA, Wist EA, Lonning PE. Trastuzumab in adjuvant breast cancer therapy: a model based cost-effectiveness analysis. Acta Oncol. 2007;46:153–64.
Stevenson MD, Oakley J, Chilcott JB. Gaussian process modeling in conjunction with individual patient simulation modeling: a case study describing the calculation of cost-effectiveness ratios for the treatment of established osteoporosis. Med Decis Making. 2004;24:89–100.
Danova M, Chiroli S, Rosti G, Doan QV. Cost-effectiveness of pegfilgrastim versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori. 2009;95:219–26.
Lyman GH, Lalla A, Barron RL, Dubois RW. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther. 2009;31:1092–104.
Ramsey SD, Liu Z, Boer R, et al. Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health. 2009;12:217–25.
Martin SC, Gagnon DD, Zhang L, Bokemeyer C, Van Marwijk Kooy M, van Hout B. Cost-utility analysis of survival with epoetin-alfa versus placebo in stage IV breast cancer. Pharmacoeconomics. 2003;21:1153–69.
Meadows ES, Klein R, Rousculp MD, Smolen L, Ohsfeldt RL, Johnston JA. Cost-effectiveness of preventative therapies for postmenopausal women with osteopenia. BMC Womens Health. 2007;7:6.
Risebrough NA, Verma S, Trudeau M, Mittmann N. Cost-effectiveness of switching to exemestane versus continued tamoxifen as adjuvant therapy for postmenopausal women with primary breast cancer. Cancer. 2007;110:499–508.
Rojnik K, Naversnik K, Mateovic-Rojnik T, Primiczakelj M. Probabilistic cost-effectiveness modeling of different breast cancer screening policies in Slovenia. Value Health. 2008;11:139–48.
Bosch JL, Beinfeld MT, Muller JE, Brady T, Gazelle GS. A cost-effectiveness analysis of a hypothetical catheter-based strategy for the detection and treatment of vulnerable coronary plaques with drug-eluting stents. J Interv Cardiol. 2005;18:339–49.
Buskens E, Nederkoorn PJ, Buijs-Van Der Woude T, et al. Imaging of carotid arteries in symptomatic patients: cost-effectiveness of diagnostic strategies. Radiology. 2004;233:101–12.
Chambers MG, Koch P, Hutton J. Development of a decision-analytic model of stroke care in the United States and Europe. Value Health. 2002;5:82–97.
Chan PS, Nallamothu BK, Gurm HS, Hayward RA, Vijan S. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation. 2007;115:2398–409.
Cram P, Vijan S, Katz D, Fendrick AM. Cost-effectiveness of in-home automated external defibrillators for individuals at increased risk of sudden cardiac death. J Gen Intern Med. 2005;20:251–8.
Derdeyn CP, Gage BF, Grubb RL Jr, Powers WJ. Cost-effectiveness analysis of therapy for symptomatic carotid occlusion: PET screening before selective extracranial-to-intracranial bypass versus medical treatment. J Nucl Med. 2000;41:800–7.
Desbiens NA. Deciding on anticoagulating the oldest old with atrial fibrillation: insights from cost-effectiveness analysis. J Am Geriatr Soc. 2002;50:863–9.
Greving JP, Buskens E, Koffijberg H, Algra A. Cost-effectiveness of aspirin treatment in the primary prevention of cardiovascular disease events in subgroups based on age, gender, and varying cardiovascular risk. Circulation. 2008;117:2875–83.
Henriksson M, Lundgren F, Carlsson P. Cost-effectiveness of endarterectomy in patients with asymptomatic carotid artery stenosis. Br J Surg. 2008;95:714–20.
Janssen MP, de Borst GJ, Mali WP, et al. Carotid stenting versus carotid endarterectomy: evidence basis and cost implications. Eur J Vasc Endovasc Surg. 2008;36:258–64.
Jonsson B, Carides GW, Burke TA, et al. Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial. J Hypertens. 2005;23:1425–31.
Karnon J, Holmes MW, Williams R, Bakhai A, Brennan A. A cost-utility analysis of clopidogrel in patients with ST elevation acute coronary syndromes in the UK. Int J Cardiol. 2010;140:315–22.
Kreisz FP, Merlin T, Moss J, Atherton J, Hiller JE, Gericke CA. The pre-test risk stratified cost-effectiveness of 64-slice computed tomography coronary angiography in the detection of significant obstructive coronary artery disease in patients otherwise referred to invasive coronary angiography. Heart Lung Circ. 2009;18:200–7.
Latimer N, Lord J, Grant RL, et al. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538.
Marchetti M, Quaglini S, Barosi G. Cost-effectiveness of screening and extended anticoagulation for carriers of both factor V Leiden and prothrombin G20210A. QJM. 2001;94:365–72.
Maud A, Lakshminarayan K, Suri MF, Vazquez G, Lanzino G, Qureshi AI. Cost-effectiveness analysis of endovascular versus neurosurgical treatment for ruptured intracranial aneurysms in the United States. J Neurosurg. 2009;110:880–6.
Mayer SA, Copeland D, Bernardini GL, et al. Cost and outcome of mechanical ventilation for life-threatening stroke. Stroke. 2000;31:2346–53.
Meenan RT, Saha S, Chou R, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischemic attack. Med Decis Making. 2007;27:161–77.
Newman J, Grobman WA, Greenland P. Combination polypharmacy for cardiovascular disease prevention in men: a decision analysis and cost-effectiveness model. Prev Cardiol. 2008;11:36–41.
Patrick AR, Avorn J, Choudhry NK. Cost-effectiveness of genotype-guided warfarin dosing for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2009;2:429–36.
Post PN, Kievit J, van Baalen JM, van den Hout WB, van Bockel JH. Routine duplex surveillance does not improve the outcome after carotid endarterectomy: a decision and cost utility analysis. Stroke. 2002;33:749–55.
Quilici S, Martin M, McGuire A, Zoellner Y. A cost-effectiveness analysis of n-3 PUFA (Omacor) treatment in post-MI patients. Int J Clin Pract. 2006;60:922–32.
Saito I, Kobayashi M, Matsushita Y, Mori A, Kawasugi K, Saruta T. Cost-utility analysis of antihypertensive combination therapy in Japan by a Monte Carlo simulation model. Hypertens Res. 2008;31:1373–83.
Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CC. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke. 2009;40:24–9.
Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: clopidogrel plus aspirin versus aspirin alone. Ann Intern Med. 2005;142:251–9.
Scuffham PA, Tippett V. The cost-effectiveness of thrombolysis administered by paramedics. Curr Med Res Opin. 2008;24:2045–58.
Sorensen SV, Dewilde S, Singer DE, Goldhaber SZ, Monz BU, Plumb JM. Cost-effectiveness of warfarin: trial versus “real-world” stroke prevention in atrial fibrillation. Am Heart J. 2009;157:1064–73.
Stahl JE, Furie KL, Gleason S, Gazelle GS. Stroke: effect of implementing an evaluation and treatment protocol compliant with NINDS recommendations. Radiology. 2003;228:659–68.
Young KC, Awad NA, Johansson M, Gillespie D, Singh MJ, Illig KA. Cost-effectiveness of abdominal aortic aneurysm repair based on aneurysm size. J Vasc Surg. 2010;51:27–32.
Aujesky D, Smith KJ, Roberts MS. Oral anticoagulation strategies after a first idiopathic venous thromboembolic event. Am J Med. 2005;118:625–35.
Bravo Vergel Y, Palmer S, Asseburg C, et al. Is primary angioplasty cost effective in the UK? Results of a comprehensive decision analysis. Heart. 2007;93:1238–43.
Gerson LB, Triadafilopoulos G, Gage BF. The management of anticoagulants in the periendoscopic period for patients with atrial fibrillation: a decision analysis. Am J Med. 2004;116:451–9.
Marchetti M, Pistorio A, Barosi G. Extended anticoagulation for prevention of recurrent venous thromboembolism in carriers of factor V Leiden–cost-effectiveness analysis. Thromb Haemost. 2000;84:752–7.
Perone N, Bounameaux H, Perrier A. Comparison of four strategies for diagnosing deep vein thrombosis: a cost-effectiveness analysis. Am J Med. 2001;110:33–40.
Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women: a cost-utility analysis. Arch Intern Med. 2007;167:290–5.
Quinn RR, Naimark DM, Oliver MJ, Bayoumi AM. Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis. 2007;50:421–32.
Sarasin FP, Gaspoz JM, Bounameaux H. Cost-effectiveness of new antiplatelet regimens used as secondary prevention of stroke or transient ischemic attack. Arch Intern Med. 2000;160:2773–8.
Sinclair SE, Frighetto L, Loewen PS, et al. Cost-utility analysis of tissue plasminogen activator therapy for acute ischaemic stroke: a Canadian healthcare perspective. Pharmacoeconomics. 2001;19:927–36.
U-King-Im JM, Hollingworth W, Trivedi RA, et al. Cost-effectiveness of diagnostic strategies prior to carotid endarterectomy. Ann Neurol. 2005;58:506–15.
Regier DA, Sunderji R, Lynd LD, Gin K, Marra CA. Cost-effectiveness of self-managed versus physician-managed oral anticoagulation therapy. CMAJ. 2006;174:1847–52.
Acknowledgments
The authors would like to acknowledge funding from Perlegen Sciences, Inc. and Genetic Technologies, Ltd.
Conflict of interest
Richard Allman is an employee of Genetic Technologies, Ltd. which offers the BREVAGen test discussed in this manuscript. David Hinds and Bryan Walser are former employees of Perlegen Sciences, Inc. Before ceasing operations in 2009, Perlegen Sciences developed a predecessor to the BREVAGen risk test. David Hinds and Bryan Walser are co-inventors on a patent describing this breast cancer risk assessment test. Tuan Dinh is an employee and Linda Green is a former employee of Archimedes, Inc., which had consulting relationships with Perlegen Sciences, Inc. and Genetic Technologies, Ltd.
Author contributions
Linda Green designed the mathematical model of tamoxifen, breast cancer, and adverse events, carried out computer simulations and data analysis, and helped draft the manuscript. Tuan Dinh helped design the mathematical model of tamoxifen, breast cancer, and adverse events, contributed to the computer simulations and data analysis, and helped draft the manuscript. David Hinds designed the model of genetic risk, contributed to the design of the study, and helped draft the manuscript. Bryan Walser conceived of the study, contributed to its design, directed the sensitivity analysis, and helped draft the manuscript. Richard Allman made improvements to the study design and model inputs and helped draft the manuscript. All authors read and approved the final manuscript. Linda Green is the guarantor for the overall content.
Author information
Authors and Affiliations
Corresponding author
Additional information
BREVAGen™ is a trademark of Genetic Technologies Ltd, Fitzroy, Victoria, Australia.
Appendix
Appendix
1.1 Calculation of Combined Risk
The combined risk of breast cancer was calculated as follows. For each risk allele i with frequency f i and per allele odds ratio r i , the risk of breast cancer due to this allele relative to the population mean risk was calculated as \( \frac{{r_{i}^{2} }}{{n_{i} }} \) for individuals with two copies of the risk allele, \( \frac{{r_{i} }}{{n_{i} }} \) for individuals with one copy of the risk allele, and \( \frac{1}{{n_{i} }} \) for individuals with no copies of the allele, where \( n_{i} = r_{i}^{2} \cdot f_{i}^{2} + 2r_{i} \cdot f_{i} \cdot (1 - f_{i} ) + 1 \cdot (1 - f_{i} )^{2}. \) The combined risk score was computed by multiplying the individual’s Gail 5-year risk by the product of these relative risks for each of the seven alleles. For example, if a woman had a Gail 5-year risk of 1.5 %, and had two copies each of the risk-bearing alleles of rs2981582 and rs3803662, one copy each of the risk-bearing alleles of rs889312, rs13387042, and rs13281615, and no copies of risk-bearing alleles of rs4415084 or rs3817198, then her combined risk score would be 1.5 %*1.31*1.31*1.05*0.99*1.01*0.88*0.96 = 2.28 %. See Table 8.
Rights and permissions
About this article
Cite this article
Green, L.E., Dinh, T.A., Hinds, D.A. et al. Economic Evaluation of Using a Genetic Test to Direct Breast Cancer Chemoprevention in White Women with a Previous Breast Biopsy. Appl Health Econ Health Policy 12, 203–217 (2014). https://doi.org/10.1007/s40258-014-0089-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-014-0089-6