Abstract
Background
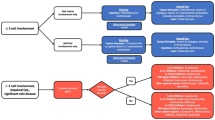
Inflammatory nail disorders can have a significant impact on patients’ quality of life owing to aesthetic and functional concerns. They are also challenging to treat because the therapeutic armamentarium is quite limited. This systematic review aims to report the efficacy and safety of Janus kinase and Tyrosine kinase 2 inhibitors in treating these conditions.
Methods
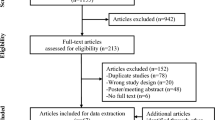
We conducted a comprehensive search on PubMed, Cochrane, and Embase Library to find eligible case reports, case series, single-arm clinical trials, and randomized controlled trials. We used the following search terms from inception until 15 December, 2024: “nail” AND “jak inhibitors” OR “tofacitinib” OR “baricitinib” OR “abrocitinib” OR “ruxolitinib” OR “deuruxolitinib” OR “upadacitinib” OR “ritlecitinib” OR “deucravacitinib” (nine searches in total).
Results
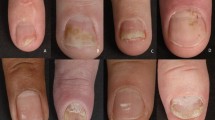
Of 441 articles found, 31 were included in this study. The most extensively studied drug was tofacitinib, followed by baricitinib, deucravacitinib, upadacitinib, and abrocitinib. Janus kinase/Tyrosine kinase 2 inhibitors demonstrated improvements in inflammatory nail conditions, with generally mild adverse events (nasopharyngitis and transient laboratory abnormalities being most common). The topical formulation of tofacitinib, the only one studied in these nail diseases, also demonstrated promising results with minimal systemic absorption and no side effects.
Conclusions
This review highlights Janus kinase/Tyrosine kinase 2 inhibitors as a valuable addition to the therapeutic arsenal for inflammatory nail disorders while emphasizing the importance of safety assessments and tailored treatment approaches. The long-term safety of Janus kinase/Tyrosine kinase 2 inhibitors still needs further investigation and the potential for adverse events emphasizes the need for tailored therapeutic strategies, including more studies on topical formulations.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Hinshaw MA, Rubin A. Inflammatory diseases of the nail unit. Semin Cutan Med Surg. 2015;34(2):109–16. https://doi.org/10.12788/j.sder.2015.0132.
Reich A, Szepietowski JC. Health-related quality of life in patients with nail disorders. Am J Clin Dermatol. 2011;12(5):313–20. https://doi.org/10.2165/11592120-000000000-00000.
Stewart CR, Algu L, Kamran R, Leveille CF, Abid K, Rae C, et al. The impact of nail psoriasis and treatment on quality of life: a systematic review. Skin Append Disord. 2021;7(2):83–9. https://doi.org/10.1159/000512688.
Belyayeva E, Gregoriou S, Chalikias J, Kontochristopoulos G, Koumantaki E, Makris M, et al. The impact of nail disorders on quality of life. Eur J Dermatol. 2013;23(3):366–71. https://doi.org/10.1684/ejd.2013.2048.
Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease. J Eur Acad Dermatol Venereol. 2014;28(12):1690–5. https://doi.org/10.1111/jdv.12368.
Iorizzo M, Vollono L, Richert B. Diagnosis and management of malignant epithelial nail unit tumors. Diagnostics (Basel). 2024;14(21):2379. https://doi.org/10.3390/diagnostics14212379.
Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–44. https://doi.org/10.1016/j.jaad.2016.12.005.
Welsch K, Holstein J, Laurence A, Ghoreschi K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol. 2017;47(7):1096–107. https://doi.org/10.1002/eji.201646680.
Huang MY, Armstrong AW. Janus-kinase inhibitors in dermatology: a review of their use in psoriasis, vitiligo, systemic lupus erythematosus, hidradenitis suppurativa, dermatomyositis, lichen planus, lichen planopilaris, sarcoidosis and graft-versus-host disease. Indian J Dermatol Venereol Leprol. 2023;90(1):30–40. https://doi.org/10.25259/ijdvl_15_2023.
Fleischmann R. Recent issues in JAK inhibitor safety: perspective for the clinician. Expert Rev Clin Immunol. 2022;18(3):295–307. https://doi.org/10.1080/1744666x.2022.2039122.
Jalles C, Lepelley M, Mouret S, Charles J, Leccia MT, Trabelsi S. Skin cancers under Janus kinase inhibitors: a World Health Organization drug safety database analysis. Therapie. 2022;77(6):649–56. https://doi.org/10.1016/j.therap.2022.04.005.
Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729–49. https://doi.org/10.1007/s13555-023-00892-5.
Ingrassia JP, Maqsood MH, Gelfand JM, Weber BN, Bangalore S, Lo Sicco KI, et al. Cardiovascular and venous thromboembolic risk with JAK inhibitors in immune-mediated inflammatory skin diseases: a systematic review and meta-analysis. JAMA Dermatol. 2024;160(1):28–36. https://doi.org/10.1001/jamadermatol.2023.4090.
Talasila S, Lee E, Teichner EM, Siegfried EC, Jackson Cullison SR. Analysis of publicly available adverse events reported for pediatric patients treated with Janus kinase inhibitors. Pediatr Dermatol. 2024;41(6):1040–6. https://doi.org/10.1111/pde.15721.
Ireland PA, Verheyden M, Jansson N, Sebaratnam D, Sullivan J. Infection risk with JAK inhibitors in dermatoses: a meta-analysis. Int J Dermatol. 2025;64(1):24–36. https://doi.org/10.1111/ijd.17501.
Bonelli M, Kerschbaumer A, Kastrati K, Ghoreschi K, Gadina M, Heinz LX, et al. Selectivity, efficacy and safety of JAKinibs: new evidence for a still evolving story. Ann Rheum Dis. 2024;83(2):139–60. https://doi.org/10.1136/ard-2023-223850.
Virtanen A, Spinelli FR, Telliez JB, O’Shea JJ, Silvennoinen O, Gadina M. JAK inhibitor selectivity: new opportunities, better drugs? Nat Rev Rheumatol. 2024;20(10):649–65. https://doi.org/10.1038/s41584-024-01153-1.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. https://doi.org/10.1136/bmj.l4898.
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–33. https://doi.org/10.11124/jbisrir-d-19-00099.
Barker TH, Habibi N, Aromataris E, Stone JC, Leonardi-Bee J, Sears K, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid Synth. 2024;22(3):378–88. https://doi.org/10.11124/jbies-23-00268.
Rózsa P, Degovics D, Baltás E, Gyulai R, Kemény L. Successful treatment of alopecia areata-associated trachyonychia with baricitinib. Int J Dermatol. 2024;63(8):1089–90. https://doi.org/10.1111/ijd.17137.
Wittmer A, De Jong K, Bolish L, Finklea L. Therapeutic response of alopecia areata-associated nail changes to baricitinib. Case Rep Dermatol Med. 2024;2024:8879884. https://doi.org/10.1155/2024/8879884.
Dhayalan A, King BA. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152(4):492–3. https://doi.org/10.1001/jamadermatol.2015.3772.
Jaller JA, Jaller JJ, Jaller AM, Jaller-Char JJ, Ferreira SB, Ferreira R, et al. Recovery of nail dystrophy potential new therapeutic indication of tofacitinib. Clin Rheumatol. 2017;36(4):971–3. https://doi.org/10.1007/s10067-017-3574-0.
Lee JS, Huh C-H, Kwon O, Yoon H-S, Cho S, Park H-S. Nail involvement in patients with moderate-to-severe alopecia areata treated with oral tofacitinib. J Dermatol Treat. 2018;29(8):819–22. https://doi.org/10.1080/09546634.2018.1466024.
Dube V. Recovery of alopecia universalis with associated nail dystrophy treated with tofacitinib: a 6-year-old child’s case report. Int J Trichol. 2021;13(6):32–3. https://doi.org/10.4103/ijt.ijt_91_21.
Asilian A, Mohammadian P, Shahmoradi Z. Effectiveness of oral tofacitinib treatment on patients with moderate-to-severe alopecia areata in Iran. J Cosmet Dermatol. 2024;23(3):886–90. https://doi.org/10.1111/jocd.16049.
He J, Yang Y. Janus kinase 1 inhibitor abrocitinib for isolated nail lichen planus: a case report and literature review. J Dermatol Treat. 2024;35(1):2434094. https://doi.org/10.1080/09546634.2024.2434094.
Chim I, Sinclair R. Effective treatment of nail lichen planus with the JAK inhibitor baricitinib. Austral J Dermatol. 2023;64(S1):49–53. https://doi.org/10.1111/ajd.14043.
He J, Weng T, Zhu W, Yang Y, Li C. Alleviation of isolated nail lichen planus by the JAK1/2 inhibitor baricitinib: a case report. J Dermatol Treat. 2023;34(1):2274816. https://doi.org/10.1080/09546634.2023.2274816.
Pünchera J, Laffitte E. Treatment of severe nail lichen planus with baricitinib. JAMA Dermatol. 2022;158(1):107–8. https://doi.org/10.1001/jamadermatol.2021.5082.
Iorizzo M, Haneke E. Tofacitinib as treatment for nail lichen planus associated with alopecia universalis. JAMA Dermatol. 2021;157(3):352–3. https://doi.org/10.1001/jamadermatol.2020.4555.
Huang J, Shi W. Successful treatment of nail lichen planus with tofacitinib: a case report and review of the literature. Front Med (Lausanne). 2023;10:1301123. https://doi.org/10.3389/fmed.2023.1301123.
Eisman S, Blauvelt A, Rich P, Sofen H, Lambert J, Merola J, et al. Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in scalp, nail, and palmoplantar psoriasis: subgroup analyses of the phase 3 POETYK PSO- 1 and PSO- 2 trials. Austral J Dermatol. 2023;64(S1):49–53. https://doi.org/10.1111/ajd.14043.
Imafuku S, Okubo Y, Tada Y, Ohtsuki M, Shao Y, Colston E, et al. 43872 Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in scalp, nail, and palmoplantar psoriasis in Japanese patients with plaque psoriasis: subset analysis of the phase 3 POETYK PSO-4 trial. J Am Acad Dermatol. 2023;89(3):AB147. https://doi.org/10.1016/j.jaad.2023.07.591.
Zhang J, Ding Y, Wang P, Li L, Pan W, Lu Y, et al. Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in patients from China mainland, Taiwan, and South Korea with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. Br J Dermatol. 2025;192(3):402–9. https://doi.org/10.1093/bjd/ljae406.
Hagino T, Onda M, Saeki H, Fujimoto E, Kanda N. Effectiveness of deucravacitinib for genital, nail and scalp lesions in patients with psoriasis: a 24-week real-world study. Clin Exp Dermatol. 2025;50(1):130–3. https://doi.org/10.1093/ced/llae312.
Wang J, Derrick K, Heilman E, Jagdeo J. Successful treatment of severe dystrophic nail psoriasis with deucravacitinib. Cutis. 2024;114(6):196–8. https://doi.org/10.12788/cutis.1142.
Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79-87.e1. https://doi.org/10.1016/j.jaad.2017.01.053.
Zhang J, Tsai T-F, Lee M-G, Zheng M, Wang G, Jin H, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88(1):36–45. https://doi.org/10.1016/j.jdermsci.2017.05.004.
Li C, Li Z, Cao Y, Li L, Li F, Li Y, et al. Tofacitinib for the treatment of nail lesions and palmoplantar pustulosis in synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. JAMA Dermatol. 2021;157(1):74–8. https://doi.org/10.1001/jamadermatol.2020.3095.
Berbert Ferreira R, Berbert Ferreira S, Neves Neto AC, Caparroz-Assef SM, Brichta L, Damiani G, et al. Topical tofacitinib as effective therapy in patients with plaque psoriasis responsive to systemic drugs but with resistant nail psoriasis. Skin Append Disord. 2023;9(5):380–4. https://doi.org/10.1159/000531119.
Wang N, Yang Q, Liu Y, Liu H. Upadacitinib in nail psoriasis: a case report. J Dermatol Treat. 2023;34(1):2246604. https://doi.org/10.1080/09546634.2023.2246604.
Muto Y, Ueki M, Asano Y. Refractory acrodermatitis continua of Hallopeau successfully treated by baricitinib: a case report. J Dermatol. 2024. https://doi.org/10.1111/1346-8138.17537.
Yang N, Wu Z, Zhang L, Mao F, Wu H, Ren Y, et al. Successful treatment of acrodermatitis continua of Hallopeau coexisting with flexural psoriasis with upadacitinib: a case report. Clin Exp Dermatol. 2024. https://doi.org/10.1093/ced/llae396.
Shen D, Jin D, Lin Z, Meng F, Li C, Ying Z. Is tofacitinib monotherapy also effective for the treatment of acrodermatitis continua of Hallopeau? Austral J Dermatol. 2024;65(8):e283–5. https://doi.org/10.1111/ajd.14384.
Chen X, Huang X, Ma L, Wang B, Tian J, Liu X. Acrodermatitis continua of Hallopeau with insufficient response to ixekizumab and infliximab, successfully treated with a combination of ustekinumab and tofacitinib. Eur J Dermatol. 2023;33(2):182–3. https://doi.org/10.1684/ejd.2023.4461.
Kan Y, Uhara H. Successful treatment of acrodermatitis continua of Hallopeau by TYK2 inhibitor with every other day. J Dermatol Treat. 2024;35(1):2316239. https://doi.org/10.1080/09546634.2024.2316239.
Yu Y, Zhao Q, Lin J, Wang J, Wang H. Treatment of refractory hand and foot eczema with nail involvement using upadacitinib: a case report. Dermatitis. 2024. https://doi.org/10.1089/derm.2023.0394.
Dube V, Bhushan R. Tofacitinib for the treatment of twenty-nail dystrophy: a single case report. Indian J Dermatol. 2022;67(6):725–7. https://doi.org/10.4103/ijd.ijd_492_22.
Zhong X, Liu T, Wei L, Bai J, Fang H, Qiao J. Tofacitinib for the treatment of trachyonychia. Am J Ther. 2024;31(4):e468–70. https://doi.org/10.1097/mjt.0000000000001676.
Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Append Disord. 2017;2(3–4):109–15. https://doi.org/10.1159/000449063.
Rigopoulos D, Baran R, Chiheb S, Daniel CR 3rd, Di Chiacchio N, Gregoriou S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81(1):228–40. https://doi.org/10.1016/j.jaad.2019.01.072.
Iorizzo M, Tosti A, Starace M, Baran R, Daniel CR 3rd, Di Chiacchio N, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83(6):1717–23. https://doi.org/10.1016/j.jaad.2020.02.056.
Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80(6):e177–8. https://doi.org/10.1016/j.jaad.2018.11.065.
Navarini AA, Burden AD, Capon F, Mrowietz U, Puig L, Köks S, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–9. https://doi.org/10.1111/jdv.14386.
Piraccini BM, Tosti A, Iorizzo M, Misciali C. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144(5):1000–5. https://doi.org/10.1046/j.1365-2133.2001.04189.x.
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–50. https://doi.org/10.1056/NEJMoa1615975.
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377(16):1525–36. https://doi.org/10.1056/NEJMoa1615977.
Mortlock RD, Ma EC, Cohen JM, Damsky W. Assessment of treatment-relevant immune biomarkers in psoriasis and atopic dermatitis: toward personalized medicine in dermatology. J Invest Dermatol. 2023;143(8):1412–22. https://doi.org/10.1016/j.jid.2023.04.005.
Nogueira M, Puig L, Torres T. JAK Inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341–52. https://doi.org/10.1007/s40265-020-01261-8.
Potestio L, Tommasino N, Lauletta G, Feo F, Ruggiero A, Martora F, et al. Efficacy and safety of deucravacitinib for the management of psoriasis: a drug safety evaluation. Expert Opin Drug Saf. 2024;23(6):677–85. https://doi.org/10.1080/14740338.2024.2351462.
Purohit VS, Ports WC, Wang C, Riley S. Systemic tofacitinib concentrations in adult patients with atopic dermatitis treated with 2% tofacitinib ointment and application to pediatric study planning. J Clin Pharmacol. 2019;59(6):811–20. https://doi.org/10.1002/jcph.1360.
Yuan L, Yu X, Shi Y, Yang B, Wang X. Acrodermatitis continua of Hallopeau: aggravating factors and treatment outcomes of 96 patients. J Dermatol Treat. 2024;35(1):2434098. https://doi.org/10.1080/09546634.2024.2434098.
Di Lernia V. Targeting the IFN-γ/CXCL10 pathway in lichen planus. Med Hypoth. 2016;92:60–1. https://doi.org/10.1016/j.mehy.2016.04.042.
Pietschke K, Holstein J, Meier K, Schäfer I, Müller-Hermelink E, Gonzalez-Menendez I, et al. The inflammation in cutaneous lichen planus is dominated by IFN-ϒ and IL-21-A basis for therapeutic JAK1 inhibition. Exp Dermatol. 2021;30(2):262–70. https://doi.org/10.1111/exd.14226.
Pelzer C, Iorizzo M. Alopecia areata of the nails: diagnosis and management. J Clin Med. 2024. https://doi.org/10.3390/jcm13113292.
Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–9. https://doi.org/10.1038/nm.3645.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of interest/competing interests
Shari R. Lipner is a consultant for Moberg Pharmaceuticals and BelleTorus Corporation. Matilde Iorizzo, Andrea Sechi, Zachary J.K. Neubauer, and Maggie Zhou have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data supporting the results of this study are presented in the paper.
Code availability
Not applicable.
Authors’ contributions
Conceptualization: MI, AS; investigation: all authors; project administration/supervision: MI, AS, SRL; writing (original draft): MI; writing (review and editing): all authors. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iorizzo, M., Sechi, A., Neubauer, Z.J.K. et al. JAK Inhibitors and Inflammatory Nail Disorders: A Systematic Review of Clinical Outcomes and Therapeutic Potential. Am J Clin Dermatol (2025). https://doi.org/10.1007/s40257-025-00946-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40257-025-00946-8