Abstract
Background
Atopic dermatitis (AD), a highly pruritic, inflammatory skin disease, affects approximately 7% of adolescents globally. A topical formulation of ruxolitinib, a Janus kinase (JAK) 1/JAK2 inhibitor, demonstrated safety and efficacy among adolescents/adults in two phase 3 studies (TRuE-AD1/TRuE-AD2).
Objective
To describe safety and efficacy of 1.5% ruxolitinib cream versus vehicle and long-term disease control of ruxolitinib cream among adolescents aged 12–17 years from pooled phase 3 study data.
Methods
Patients [≥ 12 years old with AD for ≥ 2 years, Investigator’s Global Assessment score (IGA) 2/3, and 3–20% affected body surface area (BSA) at baseline] were randomized 2:2:1 to ruxolitinib cream (0.75%/1.5%) or vehicle for 8 weeks of continuous use followed by a long-term safety (LTS) period up to 52 weeks with as-needed use. Patients originally applying vehicle were rerandomized 1:1 to 0.75%/1.5% ruxolitinib cream. Efficacy measures at week 8 included IGA treatment success (IGA-TS; i.e., score of 0/1 with ≥ 2 grade improvement from baseline), ≥ 75% improvement in Eczema Area and Severity Index (EASI-75), and ≥ 4-point improvement in itch numerical rating scale (NRS4). Measures of disease control during the LTS period included IGA score of 0 (clear) or 1 (almost clear) and percentage affected BSA. Safety was assessed throughout the study.
Results
Of 1249 randomized patients, 245 (19.6%) were aged 12–17 years. Of these, 45 patients were randomized to vehicle and 92 patients to 1.5% ruxolitinib cream. A total of 104/137 (75.9%) patients continued on 1.5% ruxolitinib cream in the LTS period [82/92 (89.1%) continued on 1.5% ruxolitinib cream; 22/45 (48.9%) patients on vehicle were reassigned to 1.5% ruxolitinib cream], and 83/104 (79.8%) of these patients completed the LTS period. At week 8, substantially more patients who applied 1.5% ruxolitinib cream versus vehicle achieved IGA-TS (50.6% versus 14.0%), EASI-75 (60.9% versus 34.9%), and NRS4 (52.1% versus 17.4%; P = 0.009). The mean (SD) reduction in itch NRS scores was significantly greater in patients applying 1.5% ruxolitinib cream versus vehicle from day 2 [− 0.9 (1.9) versus −0.2 (1.4); P = 0.03]. During the LTS period, mean (SD) trough steady-state ruxolitinib plasma concentrations at weeks 12/52 were 27.2 (55.7)/15.5 (31.5) nM. The percentage of patients achieving IGA score of 0 or 1 was sustained or further increased with 1.5% ruxolitinib cream; mean affected BSA was generally low (< 3%; i.e., mild disease). Through 52 weeks, application site reactions occurred in 1.8% of adolescent patients applying 1.5% ruxolitinib cream at any time; no patients had serious adverse events. There were no serious infections, malignancies, major adverse cardiovascular events, or thromboembolic events.
Conclusions
Meaningful anti-inflammatory and antipruritic effects were demonstrated with 1.5% ruxolitinib cream in the subset of adolescent patients with AD, comparable with those observed in the overall study population; long-term, as-needed use maintained disease control and was well tolerated.
Clinical Trial Registration
ClinicalTrials.gov identifiers NCT03745638 (registered 19 November 2018) and NCT03745651 (registered 19 November 2018).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Treatment with 1.5% ruxolitinib cream in adolescent patients with mild-to-moderate atopic dermatitis had anti-inflammatory and antipruritic effects comparable with those observed in the overall study population. |
Long-term intermittent use of ruxolitinib cream was well tolerated and provided disease control in adolescent patients with atopic dermatitis. |
1 Introduction
Atopic dermatitis (AD) is a highly pruritic, inflammatory skin disease [1] that typically begins in childhood. Globally, the prevalence of AD is approximately 7% in adolescents from 12 to < 18 years old, although reported rates vary [2,3,4]. AD can negatively impact several aspects of the quality of life (QoL) of children and adolescents, including sleep quality (e.g., sleep disturbance), school performance, and neurobehavioral function [5,6,7].
Available topical treatments for AD in adolescents include corticosteroids, calcineurin inhibitors, and phosphodiesterase-4 (PDE4) inhibitors [8, 9]. Although topical corticosteroids (TCS) are a common first-line treatment, their use is limited by potential adverse events (AEs) and toxicities that increase with long-term use [8]. In addition, topical calcineurin inhibitors (TCI) and PDE4 inhibitors have been associated with stinging and burning [8, 10]. In a retrospective study of patients applying topical therapy (including TCS, TCI, and the PDE4 inhibitor crisaborole), AD in 24% of adolescent patients was not well controlled per physician assessment. In the same study, physician- and patient-reported treatment dissatisfaction was approximately 40% and 25%, respectively [11]. Thus, there is a need for improved topical treatments for adolescents that are better tolerated and provide enhanced disease control.
Janus kinases (JAKs) modulate inflammatory cytokines involved in the pathogenesis of AD [12, 13]. Ruxolitinib is a selective inhibitor of JAK1 and JAK2 [14]; 1.5% ruxolitinib cream is approved in the USA for the treatment of patients 12 years of age or older with mild-to-moderate AD, as well as in the USA, United Kingdom, and the European Union for nonsegmental vitiligo [15,16,17]. In two phase 3 randomized, double-blind, multicenter studies of identical design [TRuE-AD1 (NCT03745638) and TRuE-AD2 (NCT03745651)], ruxolitinib cream demonstrated anti-inflammatory activity with antipruritic action versus vehicle and was well-tolerated during the 8-week vehicle-controlled (VC) period and 44-week long-term safety (LTS) period in the overall population (adolescents and adults) with AD [18, 19]. Here we report the safety and efficacy of 1.5% ruxolitinib cream (the approved formulation strength in the USA [15]) versus vehicle through week 8 and long-term disease control with 1.5% ruxolitinib cream monotherapy through week 52 in a subset of adolescents using pooled data from the two phase 3 studies.
2 Methods
2.1 Study Design and Patients
The TRuE-AD1 (NCT03745638) and TRuE-AD2 (NCT03745651) studies were conducted in ten countries in North America and Europe, and the methods have been previously published [18, 19]. Eligible patients were aged 12 years or older and had AD for more than 2 years, an Investigator’s Global Assessment (IGA) score of 2 or 3, and 3–20% affected body surface area (BSA; excluding scalp). Patients were excluded if they had an unstable course of AD, other types of eczema, immunocompromised status, or used systemic or topical therapies for AD (except bland emollients) during the washout period.
Patients were randomized (2:2:1) to either ruxolitinib cream at 0.75% twice daily (bid) or 1.5% bid, or vehicle cream bid for 8 weeks of double-blinded treatment (VC period). Patients were instructed to continue treating lesions during the VC period, even if they improved. At week 8, patients on ruxolitinib cream continued treatment for 44 additional weeks in the LTS period; patients initially randomized to vehicle were rerandomized after week 8 (1:1) to 0.75% or 1.5% ruxolitinib cream. During the LTS period, patients were instructed to treat skin areas with active AD only and stop treatment 3 days after clearance of lesions. Patients were to restart treatment with ruxolitinib cream at the first sign of recurrence. Rescue treatment was not permitted at any time during the study.
AEs of interest, those adverse events associated with oral JAK inhibitors, were assessed in patients who applied ruxolitinib cream at any time during the study. AEs of interest included notable serious infections [e.g., pneumonia, sepsis, coronavirus disease 2019 (COVID-19), and tuberculosis], malignancies, major adverse cardiovascular events (MACE), thrombosis, cytopenias (e.g., erythropenia, thrombocytopenia, and neutropenia), thrombocytosis, viral skin infections [e.g., herpes zoster (HZ), herpes simplex virus (HSV), and molluscum contagiosum], lipid elevations, and liver enzyme elevations. When AEs of interest were identified, the potential association with ruxolitinib cream was assessed through review of pharmacokinetic data available at the onset of the AE.
2.2 Assessments
Efficacy assessments in the VC period included the percentage of patients achieving IGA treatment success (IGA-TS; defined as achieving an IGA score of 0 or 1 with a ≥ 2-point improvement from baseline) and the percentage of patients achieving a ≥ 50%, ≥ 75%, and ≥ 90% improvement from baseline in Eczema Area Severity Index (EASI-50, EASI-75, and EASI-90, respectively). IGA-TS and EASI-75 responses were compared by age groups (12–17 years, 18–64 years, and ≥ 65 years) at week 8.
Itch numerical rating scale (NRS) score was reported each evening by the patients, using an electronic diary; patients were instructed to report their worst level of itch during the past 24 h period from 0 (no itch) to 10 (worst imaginable itch). The effect of ruxolitinib cream on itch was assessed by the percentage of patients achieving a ≥ 2-point (minimal clinically important difference) or ≥ 4-point (moderate difference) change in itch NRS score from baseline (itch NRS2 and itch NRS4, respectively) throughout the VC period and the time to achieve NRS2 or NRS4 [20,21,22]. Itch NRS4 response was compared by age groups at week 8. Itch was also evaluated using item 1 of the Patient-Oriented Eczema Measure (POEM) at baseline and weeks 2, 4, and 8. POEM is a seven-question quality-of-life assessment that evaluates how many days the patient was bothered by various aspects of their disease during the past 7 days [23].
The effect of ruxolitinib cream on sleep was evaluated by the percentage of patients achieving a ≥ 6-point improvement in the Patient-Reported Outcomes Measurement Information System Sleep-Related Impairment Score (PROMIS 8a) or Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score (PROMIS 8b) throughout the VC period [24].
The effect of ruxolitinib cream on QoL was assessed by mean scores in the children’s version of the Dermatology Life Quality Index (CDLQI; patients 12–15 years old) or the DLQI (patients 16 and 17 years old) at each study visit, with higher scores indicating greater impact on QoL [25].
Disease control during the LTS period was evaluated by the percentage of patients who achieved an IGA score of 0 or 1 [skin clear or almost clear of AD (i.e., no or minimal skin lesions)] and the mean percentage of BSA affected by AD.
Safety and tolerability assessments included the frequency of reported treatment-emergent adverse events (TEAEs), treatment-related adverse events (TRAEs), application site reactions, serious adverse events (SAEs), and frequency of AEs leading to treatment discontinuation. All TEAEs were graded according to Common Terminology Criteria for Adverse Events version 4.03.
Other assessments included exposure to drug during the VC period (as measured by quantity of cream used per week and steady-state trough plasma concentrations, including comparison by age groups), the time off treatment during the LTS period in patients who completed the 52-week study period (calculated as a proportion of the full LTS period (i.e., approximately 44 weeks), and the time to retreatment following complete clearing of skin lesions [IGA score of 0 at week 8 (end of the VC period)].
2.3 Statistical Analyses
Data were pooled from TRuE-AD1 and TRuE-AD2. The efficacy and disease control populations excluded patients from one study site for quality issues. The safety population included all randomized patients who applied the study drug at least once.
Statistical significance for daily itch NRS scores and for mean changes from baseline in CDLQI and DLQI were assessed using analysis of variance and covariance, respectively. The percentages of patients achieving IGA-TS, EASI-50, EASI-75, EASI-90, NRS2, NRS4, or ≥ 6-point improvement in PROMIS 8a or PROMIS 8b scores were evaluated using logistic regression, and data for IGA-TS and EASI-50/75/90 were analyzed by descriptive statistics. Patients with missing postbaseline values were imputed as nonresponders at weeks 2, 4, and 8. For the assessment of NRS2 and NRS4, only patients with baseline NRS scores ≥ 2 or ≥ 4, respectively, were included. Cumulative incidence plots were created for time to NRS2 or NRS4, and comparisons were made across treatment groups using a log-rank test. In the PROMIS 8a or 8b analyses, only patients with a PROMIS 8a or 8b score ≥ 6 at baseline were included. Disease control data (IGA 0/1 and BSA) are reported as observed.
3 Results
3.1 Patient Disposition
Of 1249 randomized patients included in the trials, a subset of 245 (19.6%) were aged 12–17 years at baseline. Of these, 45 patients were initially randomized to vehicle and 92 patients to 1.5% ruxolitinib cream; at week 8, 104/137 (75.9%) patients continued on 1.5% ruxolitinib cream [82/92 (89.1%) continued to apply 1.5% ruxolitinib cream; 22/45 (48.9%) patients originally assigned to vehicle switched to 1.5% ruxolitinib cream].
Among the 104 patients on 1.5% ruxolitinib cream in the LTS period, 83 (79.8%) completed the study (Fig. 1). The most common reasons for treatment discontinuation were withdrawal by patient [n = 15 (14.4%)] and lost to follow-up [n = 4 (3.8%)]; lack of efficacy was not reported as a primary reason for treatment discontinuation by any patient applying 1.5% ruxolitinib cream during the LTS period.
3.2 Baseline and Clinical Characteristics
Distribution of baseline demographics and clinical characteristics was similar across treatment groups (Table 1). In the 1.5% ruxolitinib cream group, median (range) age was 15.0 (12–17) years, 59 (64.1%) patients were female, 72 (78.3%) were white, and 13 (14.1%) were Black. At baseline, mean [standard deviation (SD)] affected BSA was 10.4% (6.0) and mean (SD) itch NRS score was 4.3 (2.4); 69 (75.0%) patients had a baseline IGA score of 3.
3.3 Drug Exposure and Efficacy in the VC Period
During the VC period, patients randomized to 1.5% ruxolitinib cream applied a median [interquartile range (IQR)] of 20.1 (11.8–41.1) g of cream per week. The mean (SD) trough steady-state plasma concentration (Css) of ruxolitinib averaged over weeks 2, 4, and 8 was 34.3 (48.8) nM (Fig. 2A) and mean (SD) bioavailability was 5.2% (5.7%). Mean (SD) ruxolitinib Css was similar across age groups among patients applying 1.5% ruxolitinib cream [18–64 years, 33.7 (46.4) nM; ≥ 65 years, 57.2 (112) nM].
Mean (SD) Css of ruxolitinib A by age group during the VC period and B at weeks 12 and 52 for patients 12–17 years during the LTS period. The IC50 for TPO-stimulated phosphorylated STAT3 inhibition was included from results from the phase 1 publication [14]. Css steady-state plasma concentration, IC50 half-maximal inhibitory concentration, LTS long-term safety, TPO thrombopoietin, VC vehicle-controlled
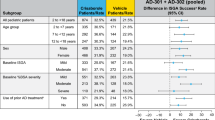
At week 8, considerably more patients on 1.5% ruxolitinib cream versus vehicle achieved IGA-TS [44/87 (50.6%) versus 6/43 (14.0%); Fig. 3A). This was also true for the secondary endpoints EASI-50 [73/87 (83.9%) versus 21/43 (48.8%); Fig. 3B), EASI-75 [53/87 (60.9%) versus 15/43 (34.9%); Fig. 3C), and EASI-90 [34/87 (39.1%) versus 3/43 (7.0%); Fig. 3D) at week 8. In addition, the efficacy outcomes of IGA-TS and EASI-75 were similar between adolescents and adults (Fig. 3E and F).
Percentage of patients achieving A IGA-TS, B EASI-50, C EASI-75, and D EASI-90 throughout the VC period and E IGA-TS and F EASI-75 by age group at week 8. EASI Eczema Area Severity Index, EASI-50 ≥ 50% improvement in EASI, EASI-75 ≥ 75% improvement in EASI, EASI-90 ≥ 90% improvement in EASI, IGA-TS Investigator’s Global Assessment-Treatment Success, VC vehicle controlled. †Defined as patients achieving an IGA score of 0 or 1 with a ≥ 2-point improvement from baseline. ‡Patients with missing postbaseline values were imputed as nonresponders at weeks 2, 4, and 8
At week 8, no clear difference in sleep quality was noted with 1.5% ruxolitinib cream versus vehicle as measured by the percentage of patients who achieved a ≥ 6-point improvement in PROMIS 8a [13/84 (15.5%) versus 6/39 (15.4%); Supplementary Fig. 1A] or PROMIS 8b score [11/82 (13.4%) versus 8/36 (22.2%); Supplementary Fig. 1B].
Mean itch NRS score decreased rapidly among patients who applied 1.5% ruxolitinib cream with evidence of a treatment effect observed as early as day 1 (approximately 12 h after first application; Fig. 4A). In addition, a significantly greater reduction in itch versus vehicle was observed on day 2, approximately 36 h after the first application of 1.5% ruxolitinib cream [mean (SD) change from baseline, − 0.9 (1.9) versus vehicle, − 0.2 (1.4); P = 0.03]. Further reductions in itch NRS scores were observed throughout 8 weeks.
Itch assessments: A mean daily itch NRS score, B percentage of patients achieving itch NRS2, C time to achieve NRS2, D percentage of patients achieving itch NRS4, E percentage of patients achieving itch NRS4 at week 8 by age group, and F time to achieve NRS4. BL baseline, NRS numerical rating scale, NRS2 ≥ 2-point improvement in itch NRS score from baseline (i.e., minimally clinically important difference), NRS4 ≥ 4-point improvement in itch NRS score from baseline (i.e., more substantial improvement). **P < 0.01 versus vehicle. ****P < 0.0001 versus vehicle. †Patients with missing postbaseline scores were imputed as nonresponders. ‡Patients in the analysis had an itch NRS score ≥ 2 at baseline. §Patients in the analysis had an itch NRS score ≥ 4 at baseline
Among patients with baseline itch NRS score ≥ 2, NRS2 by day 3 was achieved by significantly more patients who applied 1.5% ruxolitinib cream versus vehicle [28/62 (45.2%) versus 6/33 (18.2%); P = 0.009]. Most patients applying 1.5% ruxolitinib cream achieved NRS2 by week 4; this effect was maintained to week 8 (Fig. 4B). Median [95% confidence interval (CI)] time to achieve NRS2 was significantly shorter for patients who applied 1.5% ruxolitinib cream versus vehicle [3.5 (3.0, 4.0) days versus 15.5 (5.0, 51.0) days; P = 0.0002; Fig. 4C). Similarly, among patients with baseline itch NRS score ≥ 4, significantly more patients who applied 1.5% ruxolitinib cream versus vehicle achieved a substantial reduction in their itch level (NRS4) by day 4 [10/41 (24.4%) versus 1/22 (4.5%); P = 0.048]. At week 8, 25/48 (52.1%) patients treated with 1.5% ruxolitinib cream versus 4/23 (17.4%) with vehicle achieved itch NRS4 (P = 0.009; Fig. 4D). Itch NRS4 achievement was similar between adolescents and adults (Fig. 4E). Median (95% CI) time to achieve NRS4 was significantly shorter for 1.5% ruxolitinib cream versus vehicle [7.0 (5.0, 16.0) days versus 38.0 (16.0, not estimable) days; P = 0.0006; Fig. 4F). Finally, at week 8 more patients applying 1.5% ruxolitinib cream versus vehicle reported 0 days of itch in the previous 7 days, as assessed by POEM Q1 [33/84 (39.3%) versus 5/38 (13.2%); Supplementary Fig. 2].
Regarding the effect of ruxolitinib cream on adolescent patients’ QoL, mean (SD) change from baseline in CDLQI at week 8 was significantly greater for 1.5% ruxolitinib cream versus vehicle [− 6.0 (6.5) versus − 2.3 (6.3); P = 0.0012; Supplementary Fig. 3] in patients aged 12–15 years. In patients aged 16–17 years, mean (SD) change from baseline in DLQI at week 8 trended toward significance for 1.5% ruxolitinib cream versus vehicle [− 5.1 (4.3) versus −4.3 (4.6); P = 0.06].
3.4 Drug Exposure and Disease Control in the LTS Period
During the LTS period, patients who switched from vehicle to 1.5% ruxolitinib cream applied a median (IQR) of 13.6 (7.5–26.6) g of cream per week. Patients originally randomized to 1.5% ruxolitinib cream applied a median (IQR) of 14.0 (8.8–29.9) g. Mean (SD) trough Css of ruxolitinib at weeks 12 and 52 was 27.2 (55.7) nM and 15.5 (31.5) nM, respectively (Fig. 2B).
The percentage of patients with clear or almost clear skin (IGA 0/1) was sustained or further increased during the LTS period with as-needed use of ruxolitinib cream (Fig. 5A). For patients who continued on 1.5% ruxolitinib cream from the VC period, 53/77 (68.8%) had clear or almost clear skin at week 8; 48/63 (76.2%) had clear or almost clear skin at week 52. Among patients who switched from vehicle to 1.5% ruxolitinib cream during the LTS period, the percentage of patients with clear or almost clear skin was substantially increased at week 12 versus week 8 [12/20 (60.0%) versus 6/21 (28.6%], and this was sustained to week 52 [12/19 (63.2%)].
Mean affected BSA with as-needed use of ruxolitinib cream during the LTS period was low [generally < 3% (i.e., mild disease); Fig. 5B]. For patients who continued in the 1.5% ruxolitinib cream group from the VC period, mean (SD) affected BSA was 3.6% (4.3%) at week 8 and 2.2% (3.6%) at week 52. Among patients who switched from vehicle to ruxolitinib cream during the LTS period, mean (SD) affected BSA was substantially decreased at week 12 versus week 8 [5.1% (4.7%) versus 9.3% (5.7%)]; affected BSA further decreased to week 52 [1.9% (3.0%)].
In patients aged 12–15 years, mean (SD) change from the LTS period baseline in CDLQI at week 52 was − 8.5 (6.3) for patients who continued on 1.5% ruxolitinib cream from the VC period and − 3.6 (7.8) for patients who switched from vehicle to 1.5% ruxolitinib cream. In patients aged 16 to 17 years, mean (SD) change from the LTS period baseline in DLQI at week 52 was − 6.0 (4.7) for patients who continued with 1.5% ruxolitinib cream from the VC period and − 2.5 (2.4) among patients who switched from vehicle to 1.5% ruxolitinib cream.
3.5 Safety
Overall, 1.5% ruxolitinib cream was well tolerated throughout the 52-week period (Table 2), with a total of 60/114 (52.6%) patients who applied 1.5% ruxolitinib cream at any time during the study reporting a TEAE [VC period, 16/92 (17.4%) versus 17/45 (37.8%) for vehicle cream; LTS period, 55/104 (52.9%)] for an exposure-adjusted incidence rate (EAIR) of 66.8 per 100 person-years (Supplementary Table 1). No serious TEAEs were reported in patients applying 1.5% ruxolitinib cream. Treatment-related AEs were reported in 5/114 patients (4.4%; 5.6 per 100 person-years) who applied 1.5% ruxolitinib cream at any time; none occurred in greater than one patient. No patients discontinued 1.5% ruxolitinib cream owing to an AE.
Treatment-emergent application site reactions occurred in 2/114 (1.8%; 2.2 per 100 person-years) patients who received 1.5% ruxolitinib cream at any time. These were application site pain [1/114 (0.9%); 1.1 per 100 person-years; treatment-related], and application site pustules [1/114 (0.9%); 1.1 per 100 person-years].
Acne occurred in 2/114 (1.8%) patients. One patient reported worsening of acne during the VC period (grade 2 on day 4, resolved by day 51 without interruption of ruxolitinib cream; treatment-related). Another patient reported treatment-emergent papulopustular acne during the LTS period (grade 2 on day 182, resolved on day 243 without interruption of ruxolitinib cream; not treatment-related).
3.6 AEs of Interest
AEs of interest (i.e., AE reported with orally administered JAK inhibitors) occurred infrequently (Table 3). Viral infections of the skin were infrequent during the study. One patient reported grade 2 HZ in the LTS period (week 40 through week 45), which was treated with antiviral medication and resolved with continued therapy with ruxolitinib cream [19]; the HZ was not observed at application sites. The ruxolitinib plasma concentration preceding and at the time of HZ onset was 34.6 nM. No patients reported HSV or molluscum contagiosum. There were no serious infections.
Hematologic AEs were also infrequent in the study population. Neutropenia (including decreased neutrophil count) AEs were reported in two patients [developing at week 28 in one patient (plasma concentration of ruxolitinib at each visit from week 12 to 28 was below the quantification limit) and at week 9 through week 13 in the second patient (plasma concentration of ruxolitinib at week 12 was 56.5 nM)]. Both hematologic TEAEs were nonserious, were grade 1 or 2 in severity and did not require treatment interruption.
The incidence of lipid or liver enzyme elevations was low; any observed fluctuations were minor and not clinically relevant. There were no malignancies, major adverse cardiovascular events, or thromboembolic events.
4 Discussion
This analysis from two phase 3 AD studies focused on 1.5% ruxolitinib cream because it is the approved formulation strength in the USA [15] and demonstrated better efficacy than 0.75% ruxolitinib cream in the overall study population, with comparable safety and tolerability [18]. In this analysis, the efficacy of 1.5% ruxolitinib cream in adolescents (as measured by IGA-TS and EASI-75) was comparable to the overall study population during the 8-week VC period [18]. Of note, the demographics and disease characteristics of the adolescents were comparable to those of the overall study population, of which 80.4% were adults [18].
In adolescents, ruxolitinib cream also demonstrated substantially greater and faster improvement in itch compared with vehicle. As with the overall study population, significant itch improvement was observed approximately 36 h after the first application of ruxolitinib cream [26]. Although sleep disturbances have been reported in children and adolescents with AD [27, 28], no clinically meaningful improvement in sleep quality was observed during the VC period in the adolescent subpopulation, in contrast to the overall study population [18]. This may be owing to lower mean baseline PROMIS 8a and 8b scores among adolescents (16.8 and 15.8, respectively) versus the overall study population [17.3 and 18.9, respectively (Incyte, data on file)]. It is also possible that the small adolescent subpopulation means that any underlying improvement in sleep quality is unable to be detected.
In addition, adolescents achieved effective disease control with as-needed use of ruxolitinib cream during the LTS period, including those who initially applied vehicle cream (first 8 weeks), comparable with previously reported findings in the overall patient population [19]. The percentage of adolescents who completed the 52-week study was similar to that of the overall study population, potentially reflecting the effective long-term disease control experienced by patients. Furthermore, as seen in the overall study population, adolescents who switched from vehicle to ruxolitinib cream exhibited disease control comparable to adolescents who used ruxolitinib cream through the whole duration of the studies.
Adolescent patients with AD experience unique QoL effects. Patients may experience bullying and stigmatization from peers owing to their disease, further reinforcing social isolation and negative self-esteem [29, 30]. Compared with students who do not have AD, adolescent students with AD may have lower performance and worse behavior in school, with higher absenteeism (especially chronic school absenteeism) and an increased risk of learning disabilities across all severity strata [6, 7, 31, 32]. In the current study, adolescent patients who applied ruxolitinib cream had substantial improvement in QoL as measured by CDLQI and DLQI. Treatment with ruxolitinib cream may address the burden experienced by adolescents by reducing symptoms that can interfere with learning and social interaction.
The safety profile of ruxolitinib cream in adolescents throughout the 52-week study was comparable with the overall population [19]. The frequency of application site reactions was low, with application site pain and pruritus being the only events reported in more than one patient and no reports of application site folliculitis.
A previous analysis in the overall safety population found that AEs of interest were infrequent and were mostly considered unrelated to treatment [19]. In the current analysis, the only reported treatment-related AE of interest was neutropenia in one patient; this patient had neutropenia at baseline and the grade remained stable, suggesting that neutropenia may not have been directly related to the application of ruxolitinib cream. Incidence of hematologic AEs was comparable with the overall population [19]. Importantly, no notable serious infections, malignancies, major adverse cardiovascular events, or thromboembolic events occurred in adolescents applying ruxolitinib cream.
The plasma concentrations of ruxolitinib measured in this study were well below 281 nM, the half-maximal inhibitory concentration for thrombopoietin-stimulated phosphorylated STAT3 inhibition (a proxy parameter to assess JAK-related myelosuppression in bone marrow) [14, 33]. Plasma concentrations of ruxolitinib after the application of 1.5% ruxolitinib cream in the VC period were also comparable with those from adults and were lower with as-needed use of 1.5% ruxolitinib cream during the LTS period. These pharmacokinetic (PK) results, in addition to the safety data, suggest that physiologically meaningful systemic JAK inhibition is highly unlikely to occur in adolescents applying ruxolitinib cream to lesions covering up to 20% of BSA. Indeed, 1.5% ruxolitinib cream was generally well tolerated, with minimal systemic absorption even in patients with very extensive skin involvement (> 25% affected BSA at baseline) [34]. There were no remarkable findings related to the tolerability of ruxolitinib cream, including in patients applying ruxolitinib cream to sensitive areas.
Other topical treatments studied in adolescents include crisaborole [10, 35, 36] and the JAK inhibitor delgocitinib [37]. The antipruritic action of crisaborole was modest in clinical trials compared with vehicle [5, 36], and application site reactions (e.g., pain) were frequently observed [35]. Delgocitinib is approved only in Japan [38]. Unlike long-term studies with crisaborole and delgocitinib in adolescents with AD [35, 37], the current study did not allow rescue therapy, thereby enabling unconfounded assessment of the efficacy and safety of ruxolitinib cream.
Subsequent to the TRuE-AD1 and TRuE-AD2 studies, a phase 3 study in children aged 2–11 years (TRuE-AD3; NCT04921969) is currently being conducted [39]. Through the 8-week VC period of that study, safety, PK, and efficacy of 1.5% ruxolitinib cream bid were comparable with results in adolescent patients. The LTS period of the TRuE-AD3 study is currently ongoing.
A limitation of this study was the relatively low number of adolescent patients compared with the overall study population and the low number of Asian patients overall, so results may not be broadly generalizable. Another limitation is that application site reactions by body region were only partially captured. In addition, although patients were given clear instructions on how to apply ruxolitinib cream, the amount actually applied compared with what was dispensed cannot be fully determined. Adherence may wane during long-term use; however, as patients will be self-applying cream in a real-world environment, the study methodology represents actual use. Finally, patients with missing postbaseline data were imputed as nonresponders, which may result in a conservative estimate of underlying response rates.
5 Conclusions
In adolescents with AD, 1.5% ruxolitinib cream demonstrated anti-inflammatory and antipruritic effects that were comparable with those in the overall study population. Further, adolescents maintained disease control over 52 weeks with as-needed use. Ruxolitinib cream was well tolerated.
References
Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60. https://doi.org/10.1016/S0140-6736(20)31286-1.
Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417-28.e2. https://doi.org/10.1016/j.anai.2020.12.020.
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) results. Institute for Health Metrics and Evaluation, Seattle. 2020. https://vizhub.healthdata.org/gbd-results/.
Hanifin JM, Reed ML, Eczema P, Impact WG. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18(2):82–91. https://www.liebertpub.com/doi/full/10.2310/6620.2007.06034.
Legat FJ. Itch in atopic dermatitis—what is new? Front Med (Lausanne). 2021;8: 644760. https://doi.org/10.3389/fmed.2021.644760.
Cheng BT, Silverberg JI. Association of pediatric atopic dermatitis and psoriasis with school absenteeism and parental work absenteeism: a cross-sectional United States population-based study. J Am Acad Dermatol. 2021;85(4):885–92. https://doi.org/10.1016/j.jaad.2021.02.069.
Wan J, Mitra N, Hooper SR, Hoffstad OJ, Margolis DJ. Association of atopic dermatitis severity with learning disability in children. JAMA Dermatol. 2021;157(6):1–7. https://doi.org/10.1001/jamadermatol.2021.0008.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32. https://doi.org/10.1016/j.jaad.2014.03.023.
Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–44. https://doi.org/10.1111/jdv.16892.
Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503. https://doi.org/10.1016/j.jaad.2016.05.046.
Anderson P, Austin J, Lofland JH, Piercy J, Joish VN. Inadequate disease control, treatment dissatisfaction, and quality-of-life impairments among US patients receiving topical therapy for atopic dermatitis. Dermatol Ther (Heidelb). 2021;11(5):1571–85. https://doi.org/10.1007/s13555-021-00580-2.
Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat. 2013;2(3): e24137. https://doi.org/10.4161/jkst.24137.
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–28. https://doi.org/10.1016/j.cell.2017.08.006.
Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–17. https://doi.org/10.1182/blood-2009-04-214957.
Opzelura™ (ruxolitinib cream). Full prescribing information. Wilmington: Incyte Corporation; 2023.
Opzelura™ (ruxolitinib cream). Summary of product characteristics. Amsterdam: Incyte Biosciences Distribution B.V.; 2023.
Opzelura™. ruxolitinib cream. Leatherhead: Incyte Biosciences UK Ltd; 2023.
Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield L, Leung DY, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from two phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–72. https://doi.org/10.1016/j.jaad.2021.04.085.
Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Forman SB, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88(5):1008–16. https://doi.org/10.1016/j.jaad.2022.09.060.
Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–9. https://doi.org/10.1111/bjd.17744.
Silverberg JI, Lai JS, Patel KR, Singam V, Vakharia PP, Chopra R, et al. Measurement properties of the Patient-Reported Outcomes Information System (PROMIS®) Itch Questionnaire: itch severity assessments in adults with atopic dermatitis. Br J Dermatol. 2020;183(5):891–8. https://doi.org/10.1111/bjd.18978.
Reich A, Riepe C, Anastasiadou Z, Medrek K, Augustin M, Szepietowski JC, Stander S. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol. 2016;96(7):978–80. https://doi.org/10.2340/00015555-2433.
Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–9. https://doi.org/10.1001/archderm.140.12.1513.
Forrest CB, Meltzer LJ, Marcus CL, de la Motte A, Kratchman A, Buysse DJ, et al. Development and validation of the PROMIS Pediatric Sleep Disturbance and Sleep-Related Impairment item banks. Sleep. 2018;41(6):1–13. https://doi.org/10.1093/sleep/zsy054.
Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942–9. https://doi.org/10.1111/j.1365-2133.1995.tb16953.x.
Blauvelt A, Szepietowski JC, Papp K, Simpson EL, Silverberg JI, Kim BS, et al. Itch-free state in patients with atopic dermatitis treated with ruxolitinib cream: pooled analysis from two randomized phase 3 studies. J Am Acad Dermatol. 2022. https://doi.org/10.1016/j.jaad.2022.09.010.
Chang YS, Chiang BL. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci. 2016;17(4):462. https://doi.org/10.3390/ijms17040462.
Fishbein AB, Cheng BT, Tilley CC, Begolka WS, Carle AC, Forrest CB, et al. Sleep disturbance in school-aged children with atopic dermatitis: prevalence and severity in a cross-sectional sample. J Allergy Clin Immunol Pract. 2021;9(8):3120–9. https://doi.org/10.1016/j.jaip.2021.04.064.
Kelly KA, Balogh EA, Kaplan SG, Feldman SR. Skin disease in children: effects on quality of life, stigmatization, bullying, and suicide risk in pediatric acne, atopic dermatitis, and psoriasis patients. Children (Basel). 2021;8(11):1057. https://doi.org/10.3390/children8111057.
Stingeni L, Belloni Fortina A, Baiardini I, Hansel K, Moretti D, Cipriani F. Atopic dermatitis and patient perspectives: insights of bullying at school and career discrimination at work. J Asthma Allergy. 2021;14:919–28. https://doi.org/10.2147/JAA.S317009.
Vittrup I, Andersen YMF, Skov L, Wu JJ, Agner T, Thomsen SF, et al. The association between atopic dermatitis, cognitive function and school performance in children and young adults. Br J Dermatol. 2023. https://doi.org/10.1093/bjd/ljac058.
Manjunath J, Silverberg NB, Silverberg JI. Association of atopic dermatitis with poor school behaviours in US children and adolescents. J Eur Acad Dermatol Venereol. 2022;36(5):e346–8. https://doi.org/10.1111/jdv.17840.
Gong X, Chen X, Kuligowski ME, Liu X, Liu X, Cimino E, et al. Pharmacokinetics of ruxolitinib in patients with atopic dermatitis treated with ruxolitinib cream: data from phase II and III studies. Am J Clin Dermatol. 2021;22(4):555–66. https://doi.org/10.1007/s40257-021-00610-x.
Bissonnette R, Call RS, Raoof T, Zhu Z, Yeleswaram S, Gong X, Lee M. A maximum-use trial of ruxolitinib cream in adolescents and adults with atopic dermatitis. Am J Clin Dermatol. 2022;23(3):355–64. https://doi.org/10.1007/s40257-022-00690-3.
Eichenfield LF, Call RS, Forsha DW, Fowler J Jr, Hebert AA, Spellman M, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(4):641–9. https://doi.org/10.1016/j.jaad.2017.06.010.
Eichenfield LF, Yosipovitch G, Stein Gold LF, Kalabis M, Zang C, Vlahos B, et al. Improvement in disease severity and pruritus outcomes with crisaborole ointment, 2%, by baseline atopic dermatitis severity in children and adolescents with mild-to-moderate atopic dermatitis. Pediatr Dermatol. 2020;37(6):1030–7. https://doi.org/10.1111/pde.14328.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, Nagata T. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854–62. https://doi.org/10.1016/j.jaad.2021.06.014.
Japan Tobacco. JT receives approvals of CORECTIM® ointment 0.25% and CORECTIM® ointment 0.5% for the treatment of pediatric atopic dermatitis in Japan. 2021. https://www.jt.com/media/news/2021/pdf/20210323_E1.pdf. Accessed 22 Feb 2023.
Eichenfield LF, Stein Gold LF, Simpson EL, Zaenglein AL, Armstrong AW, Tollefson MM et al. A phase 3 study of ruxolitinib cream in children aged 2–<12 years with atopic dermatitis (TRuE-AD3): 8-week analysis. 32nd European Academy of Dermatology and Venereology (EADV) Congress; 2023 October 11–14; Berlin, Germany.
Acknowledgements
The authors thank the patients, investigators, and investigational sites whose participation made the study possible. This study was funded by Incyte Corporation (Wilmington, DE). Writing assistance was provided by Joshua Solomon, PhD, an employee of ICON (Blue Bell, PA), and was funded by Incyte.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Incyte Corporation (Wilmington, DE).
Conflict of interest
LFE has served as a consultant, speaker, advisory board member, or investigator for AbbVie, Amgen, Arcutis, Aslan, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Eli Lilly, Forte, Galderma, Incyte, Janssen, Johnson & Johnson, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi–Genzyme, Target RWE, and UCB. ELS is an investigator for AbbVie, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, Merck, Pfizer, and Regeneron and is a consultant with honorarium for AbbVie, Eli Lilly, Forte, Galderma, Incyte, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Sanofi Genzyme, and Valeant. KP has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite, Celgene, Coherus, Dermira, Dow Pharmaceuticals, Eli Lilly, Galderma, Gilead, GlaxoSmithKline, Incyte, InflaRx, Janssen, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, Merck (MSD), Merck Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharmaceuticals, Takeda, and UCB. JCS has served as an advisor for AbbVie, LEO Pharma, Menlo Therapeutics, Novartis, Pierre Fabre, and Trevi; has received speaker honoraria from AbbVie, Eli Lilly, Janssen-Cilag, LEO Pharma, Novartis, Sanofi-Genzyme, and Sun Pharma; and has received clinical trial funding from AbbVie, Almirall, Amgen, Galapagos, Holm, Incyte, InflaRX, Janssen-Cilag, Menlo Therapeutics, Merck, Novartis, Pfizer, Regeneron, Trevi, and UCB. AB has served as a speaker (received honoraria) for AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Regeneron, and Sanofi; served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Merck, Novartis, Pfizer, Rani, Rapt, Regeneron, Sanofi–Genzyme, Spherix Global Insights, Sun Pharma, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor; and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB Pharma. LK has served as an investigator, consultant, or speaker for AbbVie, Amgen, Anaptys, Arcutis, Dermavant, Eli Lilly, Glenmark, Incyte, Kamedis, LEO Pharma, L’Oreal, Menlo Therapeutics, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and Taro. JIS received honoraria for advisory board, speaker, and consultant services from AbbVie, Asana, Bluefin, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa, LEO Pharma, Menlo, Novartis, Pfizer, Realm, Regeneron, and Sanofi and research grants for investigator services from GlaxoSmithKline and Galderma. ECS has received consulting fees from AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi Genzyme, UCB, and Verrica; has served as a contract researcher for AI Therapeutics; has received grant funding from Pfizer; has served as an investigator for Janssen; has received institutional funding related to clinical trial sponsorship from Janssen, Lilly, Pierre Fabre, Regeneron, and Verrica; and has served as a data safety monitoring board member for LEO Pharma, Novan, Pfizer, and UCB. MEK and MEV were employees of Incyte at the time of the study. HK and HR are employees and shareholders of Incyte. ASP has served as an investigator, speaker, or data safety monitoring board member for AbbVie, Abeona, Aegerion Pharma, Azitra, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Castle Creek, Catawba, Dermavant, Eli Lilly, Galderma, InMed, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi/Genzyme, Seanergy, TWI Biotechnology, and UCB.
Availability of Data and Materials
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g., USA, Europe Union, and Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960
Ethics Approval
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocols were approved by the relevant institutional review board or ethics committee at each study site.
Consent to Participate
Written informed consent/assent was provided by all patients before enrollment.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
LFE, MEK, and MEV made substantial contributions to the conception and design of the work. LFE, ELS, KP, JCS, AB, and LK all made substantial contributions to the acquisition of data. HR made substantial contributions to the analysis of the data; all authors made substantial contributions to the interpretation of data. All authors drafted the work and revised it critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eichenfield, L.F., Simpson, E.L., Papp, K. et al. Efficacy, Safety, and Long-Term Disease Control of Ruxolitinib Cream Among Adolescents with Atopic Dermatitis: Pooled Results from Two Randomized Phase 3 Studies. Am J Clin Dermatol (2024). https://doi.org/10.1007/s40257-024-00855-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40257-024-00855-2