Abstract
Introduction
Nail changes are frequent clinical findings in patients with cutaneous psoriasis and psoriatic arthritis, often causing significant impairments in quality of life. Numerous targeted therapies have been previously studied for treatment of nail psoriasis, however, newer agents have not been captured in prior systematic reviews. With over 25 new studies published since 2020, the landscape of nail psoriasis systemic treatments is rapidly evolving, warranting analysis of recently approved therapies.
Methods
An updated systematic review of all PubMed and OVID database studies assessing efficacy and safety of targeted therapies for nail psoriasis was performed, with the goal of incorporating clinical data of recent trials and newer agents, namely brodalumab, risankizumab, and tildrakizumab. Eligibility criteria included clinical human studies reporting at least one of the nail psoriasis clinical appearance outcomes (Nail Psoriasis Severity Index, modified Nail Psoriasis Severity Index).
Results
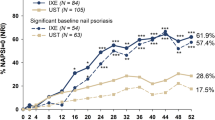
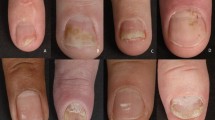
A total of 68 studies on 15 nail psoriasis targeted therapeutic agents were included. Biological agents and small molecule inhibitors included TNF-alpha inhibitors (adalimumab, infliximab, etanercept, certolizumab, golimumab), IL-17 inhibitors (ixekizumab, brodalumab, secukinumab), IL-12/23 inhibitors (ustekinumab), IL-23 inhibitors (guselkumab, risankizumab, tildrakizumab), PDE-4 inhibitors (apremilast), and JAK inhibitors (tofacitinib). These agents all demonstrated statistically significant improvements in nail outcome scores, compared with placebo or with baseline values, at weeks 10–16 and weeks 20–26, with some studies assessing efficacy up to week 60. Safety data for these agents were acceptable and consistent with known safety profiles within these timepoints, with nasopharyngitis, upper respiratory tract infections, injection site reactions, headache, and diarrhea being the most reported adverse events. Specifically, the newer agents, brodalumab, risankizumab, and tildrakizumab, showed promising outcomes for treatment of nail psoriasis on the basis of current data.
Conclusion
Numerous targeted therapies have shown significant efficacy in improving nail findings in patients with psoriasis and psoriatic arthritis. Data from head-to-head trials have shown greater efficacy of ixekizumab over adalimumab and ustekinumab, as well as brodalumab over ustekinumab, while prior meta-analyses have demonstrated superiority of ixekizumab and tofacitinib to other included agents at various assessed timepoints. Further studies on the long-term efficacy and safety of these agents, as well as randomized controlled trials involving comparison with placebo arms, are needed to fully analyze differences in efficacy of newer agents compared with previously established therapies.

Similar content being viewed by others
References
Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27. https://doi.org/10.1016/j.jaad.2005.07.073.
Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81(1):228–40. https://doi.org/10.1016/j.jaad.2019.01.072.
Chang MJ, Lee D, Desai AD, Lipner SR. The untold burden of isolated nail psoriasis: delayed diagnosis and significant risk of psoriatic arthritis in a retrospective study at an academic center [published online ahead of print, 2023 Jan 6]. J Am Acad Dermatol. 2023. https://doi.org/10.1016/j.jaad.2022.12.031.
Stewart CR, Algu L, Kamran R, et al. The impact of nail psoriasis and treatment on quality of life: a systematic review. Skin Appendage Disord. 2021;7(2):83–9. https://doi.org/10.1159/000512688.
Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–12. https://doi.org/10.1067/s0190-9622(03)00910-1.
Parrish CA, Sobera JO, Elewski BE. Modification of the Nail Psoriasis Severity Index. J Am Acad Dermatol. 2005;53(4):745–7. https://doi.org/10.1016/j.jaad.2004.11.044.
Huang IH, Wu PC, Yang TH, et al. Small molecule inhibitors and biologics in treating nail psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;85(1):135–43. https://doi.org/10.1016/j.jaad.2021.01.024.
Reich K, Conrad C, Kristensen LE, et al. Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis. J Dermatolog Treat. 2022;33(3):1652–60. https://doi.org/10.1080/09546634.2021.1892024.
Zhang X, Xie B, He Y. Efficacy of systemic treatments of nail psoriasis: a systemic literature review and meta-analysis. Front Med (Lausanne). 2021;8: 620562. https://doi.org/10.3389/fmed.2021.620562.
Hadeler E, Mosca M, Hong J, Brownstone N, Bhutani T, Liao W. Nail psoriasis: a review of effective therapies and recommendations for management. Dermatol Ther (Heidelb). 2021;11(3):799–831. https://doi.org/10.1007/s13555-021-00523-x.
Poulin Y, Crowley JJ, Langley RG, Unnebrink K, Goldblum OM, Valdecantos WC. Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH. J Eur Acad Dermatol Venereol. 2014;28(7):882–90. https://doi.org/10.1111/jdv.12198.
Leonardi C, Langley RG, Papp K, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011;147(4):429–36. https://doi.org/10.1001/archdermatol.2010.384.
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J Am Acad Dermatol. 2018;78(1):90-99.e1. https://doi.org/10.1016/j.jaad.2017.08.029.
Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–17. https://doi.org/10.1111/jdv.14015.
Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–42. https://doi.org/10.1016/j.jaad.2015.09.001.
Elewski B, Rich P, Lain E, Soung J, Lewitt GM, Jacobson A. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatolog Treat. 2022;33(1):261–5. https://doi.org/10.1080/09546634.2020.1749546.
Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73(1):48–55. https://doi.org/10.1136/annrheumdis-2013-203696.
Umezawa Y, Asahina A, Imafuku S, et al. Efficacy and safety of certolizumab pegol in Japanese patients with moderate to severe plaque psoriasis: 52-week results. Dermatol Ther (Heidelb). 2021;11(3):943–60. https://doi.org/10.1007/s13555-021-00520-0.
Mease P, Elaine Husni M, Chakravarty SD, et al. Evaluation of improvement in skin and nail psoriasis in Bio-naïve patients with active psoriatic arthritis treated with golimumab: results through week 52 of the GO-VIBRANT study. ACR Open Rheumatol. 2020;2(11):640–7. https://doi.org/10.1002/acr2.11180.
Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study [published correction appears in Arthritis Rheum.2010 Aug;62(8):2555]. Arthritis Rheum. 2009;60(4):976–86. https://doi.org/10.1002/art.24403.
Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–83. https://doi.org/10.1001/jamadermatol.2018.0793.
Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–62. https://doi.org/10.1111/1346-8138.14504.
Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–74. https://doi.org/10.1016/S0140-6736(05)67566-6.
Torii H, Nakagawa H, Japanese Infliximab Study investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59(1):40–9. https://doi.org/10.1016/j.jdermsci.2010.04.014.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87. https://doi.org/10.1136/annrheumdis-2016-209709.
Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–27. https://doi.org/10.1016/S0140-6736(17)31429-0.
Kristensen LE, Okada M, Tillett W, et al. Ixekizumab demonstrates consistent efficacy versus adalimumab in biologic disease-modifying anti-rheumatic drug-naïve psoriatic arthritis patients regardless of psoriasis severity: 52-week post hoc results from SPIRIT-H2H. Rheumatol Ther. 2022;9(1):109–25. https://doi.org/10.1007/s40744-021-00388-8.
Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–31. https://doi.org/10.1136/annrheumdis-2019-215386.
Imafuku S, Torisu-Itakura H, Nishikawa A, Zhao F, Cameron GS, Japanese UNCOVER-1 Study Group. Efficacy and safety of ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis: Subgroup analysis of a placebo-controlled, phase 3 study (UNCOVER-1). J Dermatol. 2017;44(11):1285–90. https://doi.org/10.1111/1346-8138.13927.
van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(3):477–82. https://doi.org/10.1111/jdv.14033.
Papp KA, Leonardi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3). Br J Dermatol. 2018;178(3):674–81. https://doi.org/10.1111/bjd.16050.
Langley RG, Rich P, Menter A, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2015;29(9):1763–70. https://doi.org/10.1111/jdv.12996.
Wasel N, Thaçi D, French LE, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb). 2020;10(4):663–70. https://doi.org/10.1007/s13555-020-00383-x.
Ghislain PD, Conrad C, Dutronc Y, Henneges C, Calderon DS, Vincent M, Ghys L, de Gruijter J, van de Kerkhof PCM. Comparison of ixekizumab and ustekinumab efficacy in the treatment of nail lesions of patients with moderate-to-severe plaque psoriasis: 24-week data from a phase 3 trial. Arthritis Rheumatol. 2017; 69
Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225–31. https://doi.org/10.1136/annrheumdis-2021-221019.
Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181(5):954–66. https://doi.org/10.1111/bjd.17351.
Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79-87.e1. https://doi.org/10.1016/j.jaad.2017.01.053.
Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–52. https://doi.org/10.1111/j.1346-8138.2011.01347.x.
Rich P, Bourcier M, Sofen H, et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. Br J Dermatol. 2014;170(2):398–407. https://doi.org/10.1111/bjd.12632.
Kyriakou A, Patsatsi A, Sotiriadis D. Anti-TNF agents and nail psoriasis: a single-center, retrospective, comparative study. J Dermatolog Treat. 2013;24(3):162–8. https://doi.org/10.3109/09546634.2011.646939.
Ozmen I, Erbil AH, Koc E, Tunca M. Treatment of nail psoriasis with tumor necrosis factor-alpha blocker agents: an open-label, unblinded, comparative study. J Dermatol. 2013;40(9):755–6. https://doi.org/10.1111/1346-8138.12229.
Saraceno R, Pietroleonardo L, Mazzotta A, Zangrilli A, Bianchi L, Chimenti S. TNF-α antagonists and nail psoriasis: an open, 24-week, prospective cohort study in adult patients with psoriasis. Expert Opin Biol Ther. 2013;13(4):469–73. https://doi.org/10.1517/14712598.2013.736960.
Al-Mutairi N, Nour T, Al-Rqobah D. Onychomycosis in patients of nail psoriasis on biologic therapy: a randomized, prospective open label study comparing Etanercept, Infliximab and Adalimumab. Expert Opin Biol Ther. 2013;13(5):625–9. https://doi.org/10.1517/14712598.2013.783561.
Sánchez-Regaña M, Sola-Ortigosa J, Alsina-Gibert M, Vidal-Fernández M, Umbert-Millet P. Nail psoriasis: a retrospective study on the effectiveness of systemic treatments (classical and biological therapy). J Eur Acad Dermatol Venereol. 2011;25(5):579–86. https://doi.org/10.1111/j.1468-3083.2010.03938.x.
Bardazzi F, Antonucci VA, Tengattini V, Odorici G, Balestri R, Patrizi A. A 36-week retrospective open trial comparing the efficacy of biological therapies in nail psoriasis. J Dtsch Dermatol Ges. 2013;11(11):1065–70. https://doi.org/10.1111/ddg.12173.
Trovato E, Cortonesi G, Orsini C, et al. Anti-IL23for nail psoriasis in real life: results of efficacy and safety during a 52-week period. Dermatol Ther. 2022;35(7): e15506. https://doi.org/10.1111/dth.15506.
Megna M, Tommasino N, Potestio L, et al. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an Italian 28-week retrospective study. J Dermatolog Treat. 2022;33(6):2813–20. https://doi.org/10.1080/09546634.2022.2081655.
Thaçi D, Unnebrink K, Sundaram M, Sood S, Yamaguchi Y. Adalimumab for the treatment of moderate to severe psoriasis: subanalysis of effects on scalp and nails in the BELIEVE study. J Eur Acad Dermatol Venereol. 2015;29(2):353–60. https://doi.org/10.1111/jdv.12553.
Khobzey K, Liskova I, Szegedi A, et al. Effectiveness of adalimumab in the treatment of scalp and nail affection in patients with moderate to severe plaque psoriasis in routine clinical practice. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26(1):11–4. https://doi.org/10.15570/actaapa.2017.3.
Sator P, Richter L, Saxinger W, Vasiljevic M, Stingl G. Adalimumab in the treatment of moderate-to-severe chronic plaque psoriasis in patients switching from other biologics. J Eur Acad Dermatol Venereol. 2015;29(9):1742–9. https://doi.org/10.1111/jdv.12981.
Sola-Ortigosa J, Sánchez-Regaña M, Umbert-Millet P. Efficacy of adalimumab in the treatment of psoriasis: a retrospective study of 15 patients in daily practice. J Dermatolog Treat. 2012;23(3):203–7. https://doi.org/10.3109/09546634.2010.519376.
Lanna C, Zangrilli A, Bavetta M, Campione E, Bianchi L. Efficacy and safety of adalimumab in difficult-to-treat psoriasis. Dermatol Ther. 2020;33(3): e13374. https://doi.org/10.1111/dth.13374.
Kokolakis G, Bachmann F, Wolk K, Sabat R, Philipp S. Efficacy of adalimumab for nail psoriasis during 24 months of continuous therapy. Acta Derm Venereol. 2020;100(14):adv00214. https://doi.org/10.2340/00015555-3545.
Ioannides D, Antonakopoulos N, Georgiou S, et al. Effectiveness and safety of apremilast in biologic-naïve patients with moderate psoriasis treated in routine clinical practice in Greece: the APRAISAL study. J Eur Acad Dermatol Venereol. 2021;35(9):1838–48. https://doi.org/10.1111/jdv.17392.
Lanna C, Cesaroni GM, Mazzilli S, et al. Apremilast as a target therapy for nail psoriasis: a real-life observational study proving its efficacy in restoring the nail unit. J Dermatolog Treat. 2022;33(2):1097–101. https://doi.org/10.1080/09546634.2020.1801976.
Muñoz-Santos C, Sola-Ortigosa J, Vidal D, Guilabert A. Apremilast improves quality of life and ultrasonography parameters in patients with nail psoriasis: a prospective cohort study. J Dermatol. 2021;48(10):1593–6. https://doi.org/10.1111/1346-8138.16074.
Oak ASW, Ho-Pham H, Elewski BE. Improvement of 11 patients with nail psoriasis with apremilast: results of an investigator-initiated open-label study. J Am Acad Dermatol. 2020;83(6):1830–2. https://doi.org/10.1016/j.jaad.2020.05.087.
Okubo Y, Takahashi H, Hino R, et al. Efficacy and safety of apremilast in the treatment of patients with mild-to-moderate psoriasis in Japan: results from PROMINENT, a phase 3b, open-label, single-arm study. Dermatol Ther (Heidelb). 2022;12(6):1469–80. https://doi.org/10.1007/s13555-022-00747-5.
Reich K, Korge B, Magnolo N, et al. Quality-of-life outcomes, effectiveness and tolerability of apremilast in patients with plaque psoriasis and routine German dermatology care: results from LAPIS-PSO. Dermatol Ther (Heidelb). 2022;12(1):203–21. https://doi.org/10.1007/s13555-021-00658-x.
Gregoriou S, Tsiogka A, Tsimpidakis A, Nicolaidou E, Kontochristopoulos G, Rigopoulos D. Treatment of nail psoriasis with brodalumab: an open-label unblinded study. J Eur Acad Dermatol Venereol. 2021;35(4):e299–301. https://doi.org/10.1111/jdv.17055.
Dattola A, Balato A, Megna M, et al. Certolizumab for the treatment of psoriasis and psoriatic arthritis: a real-world multicentre Italian study. J Eur Acad Dermatol Venereol. 2020;34(12):2839–45. https://doi.org/10.1111/jdv.16606.
Katsambas A, Peris K, Vena G, et al. Assessing the impact of efalizumab on nail, scalp and palmoplantar psoriasis and on quality of life: results from a multicentre, open-label, phase IIIb/IV trial. Arch Drug Inf. 2009;2(4):66–70. https://doi.org/10.1111/j.1753-5174.2009.00023.x.
Takahashi MD, Chouela EN, Dorantes GL, et al. Efalizumab in the treatment of scalp, palmoplantar and nail psoriasis: results of a 24-week Latin American sStudy. Arch Drug Inf. 2010;3(1):1–8. https://doi.org/10.1111/j.1753-5174.2009.00025.x.
Ortonne JP, Paul C, Berardesca E, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168(5):1080–7. https://doi.org/10.1111/bjd.12060.
Luger TA, Barker J, Lambert J, et al. Sustained improvement in joint pain and nail symptoms with etanercept therapy in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2009;23(8):896–904. https://doi.org/10.1111/j.1468-3083.2009.03211.x.
Gerdes S, Asadullah K, Hoffmann M, et al. Real-world evidence from the non-interventional, prospective, German multicentre PERSIST study of patients with psoriasis after 1 year of treatment with guselkumab. J Eur Acad Dermatol Venereol. 2022;36(9):1568–77. https://doi.org/10.1111/jdv.18218.
Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Guselkumab is efficacious and safe in psoriasis patients who failed anti-IL17: a 52-week real-life study. J Dermatolog Treat. 2022;33(5):2560–4. https://doi.org/10.1080/09546634.2022.2036674.
Torii H, Nakano M, Yano T, Kondo K, Nakagawa H, SPREAD Study Group. Efficacy and safety of dose escalation of infliximab therapy in Japanese patients with psoriasis: results of the SPREAD study. J Dermatol. 2017;44(5):552–9. https://doi.org/10.1111/1346-8138.13698.
Rigopoulos D, Gregoriou S, Stratigos A, et al. Evaluation of the efficacy and safety of infliximab on psoriatic nails: an unblinded, nonrandomized, open-label study. Br J Dermatol. 2008;159(2):453–6. https://doi.org/10.1111/j.1365-2133.2008.08686.x.
Fabroni C, Gori A, Troiano M, Prignano F, Lotti T. Infliximab efficacy in nail psoriasis. A retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25(5):549–53. https://doi.org/10.1111/j.1468-3083.2010.03826.x.
Megna M, Cinelli E, Gallo L, Camela E, Ruggiero A, Fabbrocini G. Risankizumab in real life: preliminary results of efficacy and safety in psoriasis during a 16-week period. Arch Dermatol Res. 2022;314(6):619–23. https://doi.org/10.1007/s00403-021-02200-7.
Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Risankizumab treatment in psoriasis patients who failed anti-IL17: A 52-week real-life study. Dermatol Ther. 2022;35(7): e15524. https://doi.org/10.1111/dth.15524.
Galluzzo M, Talamonti M, Cioni A, et al. Efficacy of tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. J Clin Med. 2022;11(9):2631. https://doi.org/10.3390/jcm11092631.
Brunasso A. Nail psoriasis improvement during tildrakizumab therapy: a real-life experience. J Drugs Dermatol. 2022;21(8):914–6. https://doi.org/10.36849/JDD.6828.
Patsatsi A, Kyriakou A, Sotiriadis D. Ustekinumab in nail psoriasis: an open-label, uncontrolled, nonrandomized study. J Dermatolog Treat. 2013;24(2):96–100. https://doi.org/10.3109/09546634.2011.607796.
Rigopoulos D, Gregoriou S, Makris M, Ioannides D. Efficacy of ustekinumab in nail psoriasis and improvement in nail-associated quality of life in a population treated with ustekinumab for cutaneous psoriasis: an open prospective unblinded study. Dermatology. 2011;223(4):325–9. https://doi.org/10.1159/000334482.
Kim BR, Yang S, Choi CW, Youn SW. Comparison of NAPSI and N-NAIL for evaluation of fingernail psoriasis in patients with moderate-to-severe plaque psoriasis treated using ustekinumab. J Dermatolog Treat. 2019;30(2):123–8. https://doi.org/10.1080/09546634.2018.1476649.
Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145(8):937–42. https://doi.org/10.1001/archdermatol.2009.175.
Berekmeri A, Mahmood F, Wittmann M, Helliwell P. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev Clin Immunol. 2018;14(9):719–30. https://doi.org/10.1080/1744666X.2018.1512404.
Golbari NM, Basehore BM, Zito PM. Brodalumab. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24(4):420–3. https://doi.org/10.1111/j.1468-3083.2009.03426.x.
Ricardo JW, Qiu Y, Lipner SR. Racial, ethnic, and sex disparities in nail psoriasis clinical trials: a systematic review. Skin Appendage Disord. 2022;8(3):171–8. https://doi.org/10.1159/000520469.
Lipner SR. A major win for the treatment of nail psoriasis. J Drugs Dermatol. 2017;16(8):731–2.
Ventura A, Mazzeo M, Gaziano R, Galluzzo M, Bianchi L, Campione E. New insight into the pathogenesis of nail psoriasis and overview of treatment strategies. Drug Des Dev Ther. 2017;11:2527–35. https://doi.org/10.2147/DDDT.S136986.
Rigopoulos D, Rompoti N, Tsiogka A, Lipner SR. How to choose a systemic treatment for moderate-to-severe nail psoriasis. J Eur Acad Dermatol Venereol. 2022;36(12):e1034–41. https://doi.org/10.1111/jdv.18435.
Ricardo JW, Lipner SR. Nail psoriasis in older adults: epidemiology, diagnosis, and topical therapy. Dermatol Clin. 2021;39(2):183–93. https://doi.org/10.1016/j.det.2020.12.011.
Zhou X-Y, Zhang J-A, Chen Ku. Nail psoriasis: treatment options and management strategies in special patient populations. Int J Dermatol Venereol. 2022;5(1):32–9. https://doi.org/10.1097/JD9.0000000000000187.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest/competing interests
Authors JKH and JWR have no conflicts of interest to declare. Author SRL has served as a consultant for Hoth Therapeutics, Ortho-Dermatologics, Belle Torus Corporation, and Moberg Pharmaceuticals.
Funding
No sources of funding were utilized for this study/manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Authors JKH, JWR, and SRL have all made substantial contributions to aspects of the work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hwang, J.K., Ricardo, J.W. & Lipner, S.R. Efficacy and Safety of Nail Psoriasis Targeted Therapies: A Systematic Review. Am J Clin Dermatol 24, 695–720 (2023). https://doi.org/10.1007/s40257-023-00786-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00786-4