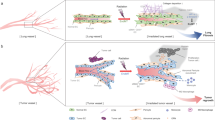
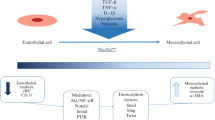
Abstract
The use of chemotherapeutic agents is becoming more frequent as the proportion of new oncology patients increases worldwide, with prolonged survival after treatment. As one of the most popular chemotherapy drugs, doxorubicin plays a substantial role in the treatment of tumors. Unfortunately, the use of doxorubicin is associated with several adverse effects, particularly severe cardiotoxicity that can be life-threatening, which greatly limits its clinical use. For decades, scientists have tried to explore many cardioprotective agents and therapeutic approaches, but their efficacy remains controversial, and some drugs have even brought about significant adverse effects. The concrete molecular mechanism of doxorubicin-induced cardiotoxicity is still to be unraveled, yet endothelial damage is gradually being identified as an important mechanism triggering the development and progression of doxorubicin-induced cardiotoxicity. Endothelial-to-mesenchymal transition (EndMT), a fundamental process regulating morphogenesis in multicellular organisms, is recognized to be associated with endothelial damage repair and acts as an important factor in the progression of cardiovascular diseases, tumors, and rheumatic immune diseases. Mounting evidence suggests that endothelial–mesenchymal transition may play a non-negligible role in doxorubicin-induced cardiotoxicity. In this paper, we reviewed the molecular mechanisms and signaling pathways of EndMT and outlined the molecular mechanisms of doxorubicin-induced cardiotoxicity and the current therapeutic advances. Furthermore, we summarized the basic principles of doxorubicin-induced endothelial–mesenchymal transition that lead to endothelial dysfunction and cardiotoxicity, aiming to provide suggestions or new ideas for the prevention and treatment of doxorubicin-induced endothelial and cardiac injury.



Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Najafi M, Hooshangi Shayesteh MR, Mortezaee K, et al. The role of melatonin on doxorubicin-induced cardiotoxicity: a systematic review. Life Sci. 2020;241: 117173. https://doi.org/10.1016/j.lfs.2019.117173.
Young RC, Ozols RF, Myers CE. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305(3):139–53. https://doi.org/10.1056/NEJM198107163050305.
Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol. 2020;29: 101394. https://doi.org/10.1016/j.redox.2019.101394.
Damiani RM, Moura DJ, Viau CM, et al. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90(9):2063–76. https://doi.org/10.1007/s00204-016-1759-y.
Polgár L, Lajkó E, Soós P, et al. Drug targeting to decrease cardiotoxicity—determination of the cytotoxic effect of GnRH-based conjugates containing doxorubicin, daunorubicin and methotrexate on human cardiomyocytes and endothelial cells. Beilstein J Org Chem. 2018;14:1583–94. https://doi.org/10.3762/bjoc.14.136.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–79. https://doi.org/10.1002/cncr.11407.
Ma W, Wei S, Zhang B, et al. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front Cell Dev Biol. 2020;8:434. https://doi.org/10.3389/fcell.2020.00434.
Sonowal H, Pal P, Shukla K, et al. Aldose reductase inhibitor, fidarestat prevents doxorubicin-induced endothelial cell death and dysfunction. Biochem Pharmacol. 2018;150:181–90. https://doi.org/10.1016/j.bcp.2018.02.018.
Tombor LS, Dimmeler S. Why is endothelial resilience key to maintain cardiac health? Basic Res Cardiol. 2022;117(1):35. https://doi.org/10.1007/s00395-022-00941-8.
Luu AZ, Chowdhury B, Al-Omran M, et al. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl Sci. 2018;3(6):861–70. https://doi.org/10.1016/j.jacbts.2018.06.005.
Todorova VK, Wei JY, Makhoul I. Subclinical doxorubicin-induced cardiotoxicity update: role of neutrophils and endothelium. Am J Cancer Res. 2021;11(9):4070–91.
Lipshultz SE, Franco VI, Sallan SE, et al. Dexrazoxane for reducing anthracycline-related cardiotoxicity in children with cancer: an update of the evidence. Prog Pediatr Cardiol. 2014;36(1–2):39–49. https://doi.org/10.1016/j.ppedcard.2014.09.007.
Benjamin RS, Minotti G. Doxorubicin–dexrazoxane from day 1 for soft-tissue sarcomas: the road to cardioprotection. Clin Cancer Res. 2021;27(14):3809–11. https://doi.org/10.1158/1078-0432.Ccr-21-1376.
Xu A, Deng F, Chen Y, et al. NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2020;130: 110525. https://doi.org/10.1016/j.biopha.2020.110525.
Tsai TH, Lin CJ, Hang CL, et al. Calcitriol attenuates doxorubicin-induced cardiac dysfunction and inhibits endothelial-to-mesenchymal transition in mice. Cells. 2019. https://doi.org/10.3390/cells8080865.
Wesseling M, Sakkers TR, de Jager SCA, et al. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vascul Pharmacol. 2018;106:1–8. https://doi.org/10.1016/j.vph.2018.02.006.
Cui X, Lu YW, Lee V, et al. Venous endothelial marker COUP-TFII regulates the distinct pathologic potentials of adult arteries and veins. Sci Rep. 2015;5:16193. https://doi.org/10.1038/srep16193.
Zeng CY, Xu J, Liu X, et al. Cardioprotective roles of endothelial progenitor cell-derived exosomes. Front Cardiovasc Med. 2021;8: 717536. https://doi.org/10.3389/fcvm.2021.717536.
Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial–mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90(11):1189–96. https://doi.org/10.1161/01.res.0000021432.70309.28.
Sanchez-Duffhues G, Orlova V, Ten Dijke P. In Brief: endothelial-to-mesenchymal transition. J Pathol. 2016;238(3):378–80. https://doi.org/10.1002/path.4653.
Peng H, Li Y, Wang C, et al. ROCK1 induces endothelial-to-mesenchymal transition in glomeruli to aggravate albuminuria in diabetic nephropathy. Sci Rep. 2016;6:20304. https://doi.org/10.1038/srep20304.
Medici D, Kalluri R. Endothelial–mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol. 2012;22(5–6):379–84. https://doi.org/10.1016/j.semcancer.2012.04.004.
Dagher O, Mury P, Thorin-Trescases N, et al. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front Cardiovasc Med. 2021;8: 658400. https://doi.org/10.3389/fcvm.2021.658400.
Richards J, El-Hamamsy I, Chen S, et al. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182(5):1922–31. https://doi.org/10.1016/j.ajpath.2013.01.037.
Yao Y, Jumabay M, Ly A, et al. A role for the endothelium in vascular calcification. Circ Res. 2013;113(5):495–504. https://doi.org/10.1161/CIRCRESAHA.113.301792.
Kovacic JC, Mercader N, Torres M, et al. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012;125(14):1795–808. https://doi.org/10.1161/CIRCULATIONAHA.111.040352.
Ranchoux B, Antigny F, Rucker-Martin C, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131(11):1006–18. https://doi.org/10.1161/CIRCULATIONAHA.114.008750.
Liang C, Gao L, Liu Y, et al. MiR-451 antagonist protects against cardiac fibrosis in streptozotocin-induced diabetic mouse heart. Life Sci. 2019;224:12–22. https://doi.org/10.1016/j.lfs.2019.02.059.
Platel V, Faure S, Corre I, et al. Endothelial-to-mesenchymal transition (EndoMT): roles in tumorigenesis, metastatic extravasation and therapy resistance. J Oncol. 2019;2019:8361945. https://doi.org/10.1155/2019/8361945.
Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. https://doi.org/10.1161/01.res.77.1.1.
Lagendijk AK, Szabo A, Merks RM, et al. Hyaluronan: a critical regulator of endothelial-to-mesenchymal transition during cardiac valve formation. Trends Cardiovasc Med. 2013;23(5):135–42. https://doi.org/10.1016/j.tcm.2012.10.002.
Evrard SM, Lecce L, Michelis KC, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016. https://doi.org/10.1038/ncomms11853.
Ciszewski WM, Wawro ME, Sacewicz-Hofman I, et al. Cytoskeleton reorganization in EndMT—the role in cancer and fibrotic diseases. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222111607.
Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, et al. TGF-beta-induced endothelial–mesenchymal transition in fibrotic diseases. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18102157.
Milan M, Pace V, Maiullari F, et al. Givinostat reduces adverse cardiac remodeling through regulating fibroblasts activation. Cell Death Dis. 2018;9(2):108. https://doi.org/10.1038/s41419-017-0174-5.
van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347(1):177–86. https://doi.org/10.1007/s00441-011-1222-6.
Medici D, Potenta S, Kalluri R. Transforming growth factor-beta2 promotes Snail-mediated endothelial–mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437(3):515–20. https://doi.org/10.1042/BJ20101500.
Sabbineni H, Verma A, Somanath PR. Isoform-specific effects of transforming growth factor β on endothelial-to-mesenchymal transition. J Cell Physiol. 2018;233(11):8418–28. https://doi.org/10.1002/jcp.26801.
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. https://doi.org/10.1126/scisignal.2005189.
Xu X, Tan X, Tampe B, et al. Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem. 2015;290(27):16653–64. https://doi.org/10.1074/jbc.M115.636944.
Noseda M, McLean G, Niessen K, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94(7):910–7. https://doi.org/10.1161/01.RES.0000124300.76171.C9.
Choi SH, Hong ZY, Nam JK, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin Cancer Res. 2015;21(16):3716–26. https://doi.org/10.1158/1078-0432.CCR-14-3193.
Li L, Chen L, Zang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/beta-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64(5):597–610. https://doi.org/10.1016/j.metabol.2015.01.014.
Wermuth PJ, Li Z, Mendoza FA, et al. Stimulation of transforming growth factor-beta1-induced endothelial-to-mesenchymal transition and tissue fibrosis by endothelin-1 (ET-1): a novel profibrotic effect of ET-1. PLoS ONE. 2016;11(9): e0161988. https://doi.org/10.1371/journal.pone.0161988.
Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20(6):713–9. https://doi.org/10.1097/bor.0b013e3283103d27.
Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83(12):4167–71. https://doi.org/10.1073/pnas.83.12.4167.
Varga J, Jimenez SA. Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun. 1986;138(2):974–80. https://doi.org/10.1016/s0006-291x(86)80591-5.
Hwang S, Chung KW. Targeting fatty acid metabolism for fibrotic disorders. Arch Pharm Res. 2021;44(9–10):839–56. https://doi.org/10.1007/s12272-021-01352-4.
Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99(2):1281–324. https://doi.org/10.1152/physrev.00021.2018.
Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. https://doi.org/10.1016/s0092-8674(03)00432-x.
Heldin CH, Moustakas A. Role of Smads in TGFbeta signaling. Cell Tissue Res. 2012;347(1):21–36. https://doi.org/10.1007/s00441-011-1190-x.
Cortes E, Lachowski D, Robinson B, et al. Tamoxifen mechanically reprograms the tumor microenvironment via HIF-1A and reduces cancer cell survival. EMBO Rep. 2019. https://doi.org/10.15252/embr.201846557.
Xiong A, Liu Y. Targeting hypoxia inducible factors-1alpha as a novel therapy in fibrosis. Front Pharmacol. 2017;8:326. https://doi.org/10.3389/fphar.2017.00326.
Deng W, Feng X, Li X, et al. Hypoxia-inducible factor 1 in autoimmune diseases. Cell Immunol. 2016;303:7–15. https://doi.org/10.1016/j.cellimm.2016.04.001.
Zhang B, Niu W, Dong HY, et al. Hypoxia induces endothelial–mesenchymal transition in pulmonary vascular remodeling. Int J Mol Med. 2018;42(1):270–8. https://doi.org/10.3892/ijmm.2018.3584.
Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126(Pt 10):2135–40. https://doi.org/10.1242/jcs.127308.
Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23(4):450–7. https://doi.org/10.1016/j.semcdb.2012.01.010.
Chang AC, Garside VC, Fournier M, et al. A Notch-dependent transcriptional hierarchy promotes mesenchymal transdifferentiation in the cardiac cushion. Dev Dyn. 2014;243(7):894–905. https://doi.org/10.1002/dvdy.24127.
Chang AC, Fu Y, Garside VC, et al. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell. 2011;21(2):288–300. https://doi.org/10.1016/j.devcel.2011.06.022.
Lin QQ, Zhao J, Zheng CG, et al. Roles of notch signaling pathway and endothelial–mesenchymal transition in vascular endothelial dysfunction and atherosclerosis. Eur Rev Med Pharmacol Sci. 2018;22(19):6485–91. https://doi.org/10.26355/eurrev_201810_16062.
Zhang Y, Dong Y, Xiong Z, et al. Sirt6-mediated endothelial-to-mesenchymal transition contributes toward diabetic cardiomyopathy via the Notch1 signaling pathway. Diabetes Metab Syndr Obes. 2020;13:4801–8. https://doi.org/10.2147/dmso.S287287.
Wang W, Wang Z, Tian D, et al. Integrin β3 mediates the endothelial-to-mesenchymal transition via the Notch pathway. Cell Physiol Biochem. 2018;49(3):985. https://doi.org/10.1159/000493229.
Patel J, Baz B, Wong HY, et al. Accelerated endothelial to mesenchymal transition increased fibrosis via deleting Notch signaling in wound vasculature. J Invest Dermatol. 2018;138(5):1166–75. https://doi.org/10.1016/j.jid.2017.12.004.
Liu J, Dong F, Jeong J, et al. Constitutively active Notch1 signaling promotes endothelial–mesenchymal transition in a conditional transgenic mouse model. Int J Mol Med. 2014;34(3):669–76. https://doi.org/10.3892/ijmm.2014.1818.
Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–79. https://doi.org/10.1038/nrm3470.
Bergmann C, Distler JH. Canonical Wnt signaling in systemic sclerosis. Lab Invest. 2016;96(2):151–5. https://doi.org/10.1038/labinvest.2015.154.
Foulquier S, Daskalopoulos EP, Lluri G, et al. WNT Signaling in Cardiac and Vascular Disease. Pharmacol Rev. 2018;70(1):68–141. https://doi.org/10.1124/pr.117.013896.
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99. https://doi.org/10.1016/j.cell.2017.05.016.
Li H, Zhao Q, Chang L, et al. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway. Lipids Health Dis. 2019;18(1):62. https://doi.org/10.1186/s12944-019-1006-7.
Wang Z, Wang Z, Gao L, et al. miR-222 inhibits cardiac fibrosis in diabetic mice heart via regulating Wnt/β-catenin-mediated endothelium to mesenchymal transition. J Cell Physiol. 2020;235(3):2149–60. https://doi.org/10.1002/jcp.29119.
Zhang J, Rojas S, Singh S, et al. Wnt2 contributes to the development of atherosclerosis. Front Cardiovasc Med. 2021;8: 751720. https://doi.org/10.3389/fcvm.2021.751720.
Zhang ZY, Zhai C, Yang XY, et al. Knockdown of CD146 promotes endothelial-to-mesenchymal transition via Wnt/β-catenin pathway. PLoS ONE. 2022;17(8): e0273542. https://doi.org/10.1371/journal.pone.0273542.
Cheng SL, Shao JS, Behrmann A, et al. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial–mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1679–89. https://doi.org/10.1161/ATVBAHA.113.300647.
Gvaramia D, Blaauboer ME, Hanemaaijer R, et al. Role of caveolin-1 in fibrotic diseases. Matrix Biol. 2013;32(6):307–15. https://doi.org/10.1016/j.matbio.2013.03.005.
Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276(9):6727–38. https://doi.org/10.1074/jbc.M008340200.
Li Z, Wermuth PJ, Benn BS, et al. Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am J Pathol. 2013;182(2):325–31. https://doi.org/10.1016/j.ajpath.2012.10.022.
Huang X, Pan L, Pu H, et al. Loss of caveolin-1 promotes endothelial–mesenchymal transition during sepsis: a membrane proteomic study. Int J Mol Med. 2013;32(3):585–92. https://doi.org/10.3892/ijmm.2013.1432.
Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121(22):2407–18. https://doi.org/10.1161/CIRCULATIONAHA.110.938217.
Soldano S, Paolino S, Pizzorni C, et al. Dual endothelin receptor antagonists contrast the effects induced by endothelin-1 on cultured human microvascular endothelial cells. Clin Exp Rheumatol. 2017;35(3):484–93.
Cipriani P, Di Benedetto P, Ruscitti P, et al. The endothelial–mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol. 2015;42(10):1808–16. https://doi.org/10.3899/jrheum.150088.
Piera-Velazquez S, Jimenez SA. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S7. https://doi.org/10.1186/1755-1536-5-S1-S7.
Gasperini P, Espigol-Frigole G, McCormick PJ, et al. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 2012;72(5):1157–69. https://doi.org/10.1158/0008-5472.CAN-11-3067.
Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18(8):293–8. https://doi.org/10.1016/j.tcm.2009.01.001.
Arciniegas E, Neves CY, Carrillo LM, et al. Endothelial–mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium. 2005;12(4):193–200. https://doi.org/10.1080/10623320500227283.
Lovisa S, Fletcher-Sananikone E, Sugimoto H, et al. Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal. 2020. https://doi.org/10.1126/scisignal.aaz2597.
Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–61. https://doi.org/10.1038/nm1613.
Zhang Y, Wu X, Li Y, et al. Endothelial to mesenchymal transition contributes to arsenic-trioxide-induced cardiac fibrosis. Sci Rep. 2016;6:33787. https://doi.org/10.1038/srep33787.
Glover EK, Jordan N, Sheerin NS, et al. Regulation of endothelial-to-mesenchymal transition by microRNAs in chronic allograft dysfunction. Transplantation. 2019;103(4):e64–73. https://doi.org/10.1097/tp.0000000000002589.
Chen XY, Lv RJ, Zhang W, et al. Inhibition of myocyte-specific enhancer factor 2A improved diabetic cardiac fibrosis partially by regulating endothelial-to-mesenchymal transition. Oncotarget. 2016;7(21):31053–66. https://doi.org/10.18632/oncotarget.8842.
Qin W, Zhang L, Li Z, et al. Endothelial to mesenchymal transition contributes to nicotine-induced atherosclerosis. Theranostics. 2020;10(12):5276–89. https://doi.org/10.7150/thno.42470.
Zhang S, Li Y, Huang X, et al. Seamless genetic recording of transiently activated mesenchymal gene expression in endothelial cells during cardiac fibrosis. Circulation. 2021;144(25):2004–20. https://doi.org/10.1161/circulationaha.121.055417.
Qiao X, van der Zanden SY, Wander DPA, et al. Uncoupling DNA damage from chromatin damage to detoxify doxorubicin. Proc Natl Acad Sci USA. 2020;117(26):15182–92. https://doi.org/10.1073/pnas.1922072117.
Sun Z, Schriewer J, Tang M, et al. The TGF-β pathway mediates doxorubicin effects on cardiac endothelial cells. J Mol Cell Cardiol. 2016;90:129–38. https://doi.org/10.1016/j.yjmcc.2015.12.010.
Wilkinson EL, Sidaway JE, Cross MJ. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol Open. 2016;5(10):1362–70. https://doi.org/10.1242/bio.020362.
Sawicki KT, Sala V, Prever L, et al. Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol. 2021;61:309–32. https://doi.org/10.1146/annurev-pharmtox-030620-104842.
Gaudin PB, Hruban RH, Beschorner WE, et al. Myocarditis associated with doxorubicin cardiotoxicity. Am J Clin Pathol. 1993;100(2):158–63. https://doi.org/10.1093/ajcp/100.2.158.
Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–52. https://doi.org/10.1016/j.pcad.2006.10.002.
Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–7. https://doi.org/10.7326/0003-4819-91-5-710.
Chatterjee K, Zhang J, Honbo N, et al. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–62. https://doi.org/10.1159/000265166.
Appel JM, Nielsen D, Zerahn B, et al. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol. 2007;46(5):576–80. https://doi.org/10.1080/02841860601156165.
Tian W, Yang L, Liu, et al. Resveratrol attenuates doxorubicin-induced cardiotoxicity in rats by up-regulation of vascular endothelial growth factor B. J Nutr Biochem. 2020;79: 108132. https://doi.org/10.1016/j.jnutbio.2019.01.018.
Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. https://doi.org/10.1038/nm.2919.
Shi Y, Moon M, Dawood S, et al. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36(4):296–305. https://doi.org/10.1007/s00059-011-3470-3.
Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749–63. https://doi.org/10.1161/CIRCULATIONAHA.112.100560.
Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116(7):1269–76. https://doi.org/10.1161/CIRCRESAHA.116.305381.
Oliveira MS, Melo MB, Carvalho JL, et al. Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J Cancer Sci Ther. 2013;5(2):52–7. https://doi.org/10.4172/1948-5956.1000184.
Cui N, Wu F, Lu WJ, et al. Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIα to IIβ in human stem cell derived cardiomyocytes. J Cell Mol Med. 2019;23(7):4627–39. https://doi.org/10.1111/jcmm.14346.
Choi ME, Price DR, Ryter SW, et al. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4(15): e128834. https://doi.org/10.1172/jci.insight.128834.
Wenningmann N, Knapp M, Ande A, et al. Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol Pharmacol. 2019;96(2):219–32. https://doi.org/10.1124/mol.119.115725.
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. https://doi.org/10.1016/j.cell.2012.03.042.
Meng L, Lin H, Zhang J, et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J Mol Cell Cardiol. 2019;136:15–26. https://doi.org/10.1016/j.yjmcc.2019.08.009.
Ichikawa Y, Ghanefar M, Bayeva M, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617–30. https://doi.org/10.1172/JCI72931.
Jahn SK, Hennicke T, Kassack MU, et al. Distinct influence of the anthracycline derivative doxorubicin on the differentiation efficacy of mESC-derived endothelial progenitor cells. Biochim Biophys Acta Mol Cell Res. 2020;1867(7): 118711. https://doi.org/10.1016/j.bbamcr.2020.118711.
Arta A, Larsen JB, Eriksen AZ, et al. Cell targeting strategy affects the intracellular trafficking of liposomes altering loaded doxorubicin release kinetics and efficacy in endothelial cells. Int J Pharm. 2020;588: 119715. https://doi.org/10.1016/j.ijpharm.2020.119715.
Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–90. https://doi.org/10.1161/01.RES.0000255690.03436.ae.
Räsänen M, Degerman J, Nissinen TA, et al. VEGF-B gene therapy inhibits doxorubicin-induced cardiotoxicity by endothelial protection. Proc Natl Acad Sci USA. 2016;113(46):13144–9. https://doi.org/10.1073/pnas.1616168113.
Park M, Kim J, Kim T, et al. REDD1 is a determinant of low-dose metronomic doxorubicin-elicited endothelial cell dysfunction through downregulation of VEGFR-2/3 expression. Exp Mol Med. 2021;53(10):1612–22. https://doi.org/10.1038/s12276-021-00690-z.
Shamoon L, Espitia-Corredor JA, Dongil P, et al. Resolvin E1 attenuates doxorubicin-induced endothelial senescence by modulating NLRP3 inflammasome activation. Biochem Pharmacol. 2022;201: 115078. https://doi.org/10.1016/j.bcp.2022.115078.
Pan JA, Zhang H, Lin H, et al. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 2021;46: 102120. https://doi.org/10.1016/j.redox.2021.102120.
Grakova EV, Shilov SN, Kopeva KV, et al. Anthracycline-induced cardiotoxicity: the role of endothelial dysfunction. Cardiology. 2021;146(3):315–23. https://doi.org/10.1159/000512771.
Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361. https://doi.org/10.1038/ncomms14361.
Cho JG, Lee A, Chang W, et al. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol. 2018;9:294. https://doi.org/10.3389/fimmu.2018.00294.
Yang Z, He LJ, Sun SR. Role of endothelial cells in renal fibrosis. Adv Exp Med Biol. 2019;1165:145–63. https://doi.org/10.1007/978-981-13-8871-2_8.
Chude CI, Amaravadi RK. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 2017;18(6):1279. https://doi.org/10.3390/ijms18061279.
Daiber A, Steven S, Weber A, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174(12):1591–619. https://doi.org/10.1111/bph.13517.
Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial–mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179(3):1074–80. https://doi.org/10.1016/j.ajpath.2011.06.001.
Shu DY, Wojciechowski M, Lovicu FJ. ERK1/2-mediated EGFR-signaling is required for TGFbeta-induced lens epithelial-mesenchymal transition. Exp Eye Res. 2019;178:108–21. https://doi.org/10.1016/j.exer.2018.09.021.
Perez L, Munoz-Durango N, Riedel CA, et al. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017;33:41–54. https://doi.org/10.1016/j.cytogfr.2016.09.002.
Zhu N, Zhang GX, Yi B, et al. Nur77 limits endothelial barrier disruption to LPS in the mouse lung. Am J Physiol Lung Cell Mol Physiol. 2019;317(5):L615–24. https://doi.org/10.1152/ajplung.00425.2018.
Rudd MA, Johnstone MT, Rabbani LE, et al. Thrombolytic therapy causes an increase in vascular permeability that is reversed by 1-deamino-8-d-vasopressin. Circulation. 1991;84(6):2568–73. https://doi.org/10.1161/01.cir.84.6.2568.
Li Z, Li X, Zhu Y, et al. Protective effects of acetylcholine on hypoxia-induced endothelial-to-mesenchymal transition in human cardiac microvascular endothelial cells. Mol Cell Biochem. 2020;473(1–2):101–10. https://doi.org/10.1007/s11010-020-03811-w.
Zhou K, Tian KJ, Yan BJ, et al. A promising field: regulating imbalance of EndMT in cardiovascular diseases. Cell Cycle. 2021;20(15):1477–86. https://doi.org/10.1080/15384101.2021.1951939.
Lee SW, Won JY, Kim WJ, et al. Snail as a potential target molecule in cardiac fibrosis: paracrine action of endothelial cells on fibroblasts through snail and CTGF axis. Mol Ther. 2013;21(9):1767–77. https://doi.org/10.1038/mt.2013.146.
Kuo HF, Liu IF, Li CY, et al. Endocardial endothelial dysfunction and unknown polymorphic composite accumulation in heart failure. Biomedicines. 2021;9(10):1465. https://doi.org/10.3390/biomedicines9101465.
Zhu K, Pan Q, Jia LQ, et al. MiR-302c inhibits tumor growth of hepatocellular carcinoma by suppressing the endothelial–mesenchymal transition of endothelial cells. Sci Rep. 2014;4:5524. https://doi.org/10.1038/srep05524.
Haynes BA, Yang LF, Huyck RW, et al. Endothelial-to-mesenchymal transition in human adipose tissue vasculature alters the particulate secretome and induces endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2019;39(10):2168–91. https://doi.org/10.1161/atvbaha.119.312826.
Xie Y, Liao J, Yu Y, et al. Endothelial-to-mesenchymal transition in human idiopathic dilated cardiomyopathy. Mol Med Rep. 2018;17(1):961–9. https://doi.org/10.3892/mmr.2017.8013.
Carlson BW, Craft MA, Carlson JR, et al. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40(3):325–36. https://doi.org/10.1007/s11357-018-0025-z.
Clayton ZS, Hutton DA, Mahoney SA, et al. Anthracycline chemotherapy-mediated vascular dysfunction as a model of accelerated vascular aging. Aging Cancer. 2021;2(1–2):45–69. https://doi.org/10.1002/aac2.12033.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Sun X, Nkennor B, Mastikhina O, et al. Endothelium-mediated contributions to fibrosis. Semin Cell Dev Biol. 2020;101:78–86. https://doi.org/10.1016/j.semcdb.2019.10.015.
Choi SH, Kim AR, Nam JK, et al. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat Commun. 2018;9(1):5108. https://doi.org/10.1038/s41467-018-07470-w.
Kim SH, Song Y, Seo HR. GSK-3β regulates the endothelial-to-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J Exp Clin Cancer Res. 2019;38(1):46. https://doi.org/10.1186/s13046-019-1050-1.
Yu J, Wang C, Kong Q, et al. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine. 2018;40:125–39. https://doi.org/10.1016/j.phymed.2018.01.009.
Wang AJ, Zhang J, Xiao M, et al. Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell Mol Life Sci. 2021;78(7):3105–25. https://doi.org/10.1007/s00018-020-03729-y.
Li J, Chang HM, Banchs J, et al. Detection of subclinical cardiotoxicity in sarcoma patients receiving continuous doxorubicin infusion or pre-treatment with dexrazoxane before bolus doxorubicin. Cardiooncology. 2020;6:1. https://doi.org/10.1186/s40959-019-0056-3.
Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64(9):938–45. https://doi.org/10.1016/j.jacc.2014.06.1167.
Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-hodgkin lymphoma: a report of the Children’s Oncology Group randomized trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34(8):854–62. https://doi.org/10.1200/JCO.2015.60.8851.
Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351(2):145–53. https://doi.org/10.1056/NEJMoa035153.
Leger K, Slone T, Lemler M, et al. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy. Pediatr Blood Cancer. 2015;62(1):123–7. https://doi.org/10.1002/pbc.25206.
Monahan DS, Flaherty E, Hameed A, et al. Resveratrol significantly improves cell survival in comparison to dexrazoxane and carvedilol in a h9c2 model of doxorubicin induced cardiotoxicity. Biomed Pharmacother. 2021;140: 111702. https://doi.org/10.1016/j.biopha.2021.111702.
Kopp LM, Womer RB, Schwartz CL, et al. Effects of dexrazoxane on doxorubicin-related cardiotoxicity and second malignant neoplasms in children with osteosarcoma: a report from the Children’s Oncology Group. Cardiooncology. 2019;5:15. https://doi.org/10.1186/s40959-019-0050-9.
Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61(23):2355–62. https://doi.org/10.1016/j.jacc.2013.02.072.
Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–62. https://doi.org/10.1016/j.jacc.2006.07.052.
Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013;167(5):2306–10. https://doi.org/10.1016/j.ijcard.2012.06.023.
Gulati G, Heck SL, Rosjo H, et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J Am Heart Assoc. 2017;6(11): e006513. https://doi.org/10.1161/JAHA.117.006513.
Chandran K, Aggarwal D, Migrino RQ, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96(4):1388–98. https://doi.org/10.1016/j.bpj.2008.10.042.
McGowan JV, Chung R, Maulik A, et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75. https://doi.org/10.1007/s10557-016-6711-0.
Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65(23):2511–22. https://doi.org/10.1016/j.jacc.2015.04.013.
Bischoff J. Endothelial-to-mesenchymal transition. Circ Res. 2019;124(8):1163–5. https://doi.org/10.1161/circresaha.119.314813.
Chevalier J, Yin H, Arpino JM, et al. Obstruction of small arterioles in patients with critical limb ischemia due to partial endothelial-to-mesenchymal transition. iScience. 2020;23(6): 101251. https://doi.org/10.1016/j.isci.2020.101251.
Courchaine K, Rugonyi S. Optical coherence tomography for in vivo imaging of endocardial to mesenchymal transition during avian heart development. Biomed Opt Express. 2019;10(11):5989–95. https://doi.org/10.1364/boe.10.005989.
Zhao G, Lu H, Liu Y, et al. Single-cell transcriptomics reveals endothelial plasticity during diabetic atherogenesis. Front Cell Dev Biol. 2021;9: 689469. https://doi.org/10.3389/fcell.2021.689469.
Lefrancais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–9. https://doi.org/10.1038/nature21706.
Kwok JC, Richardson DR. The cardioprotective effect of the iron chelator dexrazoxane (ICRF-187) on anthracycline-mediated cardiotoxicity. Redox Rep. 2000;5(6):317–24. https://doi.org/10.1179/135100000101535898.
Hasinoff BB, Patel D, Wu X. The role of topoisomerase IIbeta in the mechanisms of action of the doxorubicin cardioprotective agent dexrazoxane. Cardiovasc Toxicol. 2020;20(3):312–20. https://doi.org/10.1007/s12012-019-09554-5.
Swain SM, Whaley FS, Gerber MC, et al. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15(4):1333–40. https://doi.org/10.1200/JCO.1997.15.4.1333.
McCormack K. The cardioprotective effect of dexrazoxane (Cardioxane) is consistent with sequestration of poly(ADP-ribose) by self-assembly and not depletion of topoisomerase 2B. Ecancermedicalscience. 2018;12:889. https://doi.org/10.3332/ecancer.2018.889.
Sishi BJ, Loos B, van Rooyen J, et al. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem Pharmacol. 2013;85(1):124–34. https://doi.org/10.1016/j.bcp.2012.10.005.
Li M, Sala V, De Santis MC, et al. Phosphoinositide 3-kinase gamma inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation. 2018;138(7):696–711. https://doi.org/10.1161/circulationaha.117.030352.
Ding Y, Sun X, Huang W, et al. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res. 2011;109(6):658–69. https://doi.org/10.1161/circresaha.111.248260.
Tadokoro T, Ikeda M, Ide T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.132747.
He H, Wang L, Qiao Y, et al. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKalpha2 and activating adaptive autophagy. Redox Biol. 2021;48: 102185. https://doi.org/10.1016/j.redox.2021.102185.
Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116(7):2672–80. https://doi.org/10.1073/pnas.1821022116.
Mukhopadhyay P, Rajesh M, Batkai S, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296(5):H1466–83. https://doi.org/10.1152/ajpheart.00795.2008.
Cabral REL, Mendes TB, Vendramini V, et al. Carnitine partially improves oxidative stress, acrosome integrity, and reproductive competence in doxorubicin-treated rats. Andrology. 2018;6(1):236–46. https://doi.org/10.1111/andr.12426.
Rocha VC, Franca LS, de Araujo CF, et al. Protective effects of mito-TEMPO against doxorubicin cardiotoxicity in mice. Cancer Chemother Pharmacol. 2016;77(3):659–62. https://doi.org/10.1007/s00280-015-2949-7.
Clayton ZS, Brunt VE, Hutton DA, et al. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC CardioOncol. 2020;2(3):475–88. https://doi.org/10.1016/j.jaccao.2020.06.010.
Zilinyi R, Czompa A, Czegledi A, et al. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018. https://doi.org/10.3390/molecules23051184.
Liu D, Ma Z, Di S, et al. AMPK/PGC1alpha activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med. 2018;129:59–72. https://doi.org/10.1016/j.freeradbiomed.2018.08.032.
Li HR, Wang C, Sun P, et al. Melatonin attenuates doxorubicin-induced cardiotoxicity through preservation of YAP expression. J Cell Mol Med. 2020;24(6):3634–46. https://doi.org/10.1111/jcmm.15057.
Kobashigawa LC, Xu YC, Padbury JF, et al. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: an in vitro study. PLoS ONE. 2014;9(8): e104888. https://doi.org/10.1371/journal.pone.0104888.
Asensio-Lopez MC, Lax A, Pascual-Figal DA, et al. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic Biol Med. 2011;51(10):1861–71. https://doi.org/10.1016/j.freeradbiomed.2011.08.015.
Arinno A, Maneechote C, Khuanjing T, et al. Cardioprotective effects of melatonin and metformin against doxorubicin-induced cardiotoxicity in rats are through preserving mitochondrial function and dynamics. Biochem Pharmacol. 2021;192: 114743. https://doi.org/10.1016/j.bcp.2021.114743.
Xue H, Ren W, Denkinger M, et al. Nutrition modulation of cardiotoxicity and anticancer efficacy related to doxorubicin chemotherapy by glutamine and omega-3 polyunsaturated fatty acids. JPEN J Parenter Enteral Nutr. 2016;40(1):52–66. https://doi.org/10.1177/0148607115581838.
Todorova VK, Kaufmann Y, Hennings L, et al. Oral glutamine protects against acute doxorubicin-induced cardiotoxicity of tumor-bearing rats. J Nutr. 2010;140(1):44–8. https://doi.org/10.3945/jn.109.113415.
Spallarossa P, Garibaldi S, Altieri P, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37(4):837–46. https://doi.org/10.1016/j.yjmcc.2004.05.024.
Oliveira PJ, Bjork JA, Santos MS, et al. Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol. 2004;200(2):159–68. https://doi.org/10.1016/j.taap.2004.04.005.
Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. 2010;85(11):894–6. https://doi.org/10.1002/ajh.21840.
Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–8. https://doi.org/10.1161/CIRCULATIONAHA.114.013777.
Huelsenbeck J, Henninger C, Schad A, et al. Inhibition of Rac1 signaling by lovastatin protects against anthracycline-induced cardiac toxicity. Cell Death Dis. 2011;2: e190. https://doi.org/10.1038/cddis.2011.65.
Huang WP, Yin WH, Chen JS, et al. Fenofibrate attenuates doxorubicin-induced cardiac dysfunction in mice via activating the eNOS/EPC pathway. Sci Rep. 2021;11(1):1159. https://doi.org/10.1038/s41598-021-80984-4.
Shoukry HS, Ammar HI, Rashed LA, et al. Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS ONE. 2017;12(7): e0181535. https://doi.org/10.1371/journal.pone.0181535.
Zordoky BNM, Robertson IM, Dyck JRB. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochem Biophys Acta. 2015;1852(6):1155–77. https://doi.org/10.1016/j.bbadis.2014.10.016.
Avila MS, Ayub-Ferreira SM, de Barros Wanderley Jr MR, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71(20):2281–90. https://doi.org/10.1016/j.jacc.2018.02.049.
Ekinci Akdemir FN, Yildirim S, Kandemir FM, et al. Protective effects of gallic acid on doxorubicin-induced cardiotoxicity; an experimantal study. Arch Physiol Biochem. 2021;127(3):258–65. https://doi.org/10.1080/13813455.2019.1630652.
Hou J, Yun Y, Cui C, et al. Ginsenoside Rh2 mitigates doxorubicin-induced cardiotoxicity by inhibiting apoptotic and inflammatory damage and weakening pathological remodelling in breast cancer-bearing mice. Cell Prolif. 2022;55(6):e13246. https://doi.org/10.1111/cpr.13246.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Beijing Health Promotion Association (20181BCB24013) and Special Funds for Guiding Local Scientific and Technological Development from the Central Government of China (S2019CXSFG0016).
Conflict of interest
Jie Feng and Yanqing Wu have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
JF was responsible for the literature search, data compilation, and writing of the paper. YW provided guidance on the design of the article, quality control and review of the article, and was responsible for the overall responsibility, supervision and management of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, J., Wu, Y. Endothelial-to-Mesenchymal Transition: Potential Target of Doxorubicin-Induced Cardiotoxicity. Am J Cardiovasc Drugs 23, 231–246 (2023). https://doi.org/10.1007/s40256-023-00573-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00573-w