Abstract
Background
Tafamidis was approved for the treatment of hereditary and wild-type transthyretin amyloid cardiomyopathy (ATTRwt-CM) in May 2019, based on findings from the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT).
Methods
This retrospective cohort study evaluated the factors associated with tafamidis prescription after diagnosis of ATTRwt-CM in the real world. Between May 2019 and December 2020, 430 patients with 6 months’ database activity were indexed from the de-identified US Optum electronic healthcare records at first diagnosis of ATTRwt-CM or prescription of tafamidis, then followed until last activity or death. Of these, 209 patients were prescribed tafamidis during follow-up, 167 (80%) within 1 month, 98% by 6 months, and 100% by 9 months. Median time from index to tafamidis prescription, calculated using the Kaplan–Meier method, was 5.8 months (95% confidence interval [CI] 2.4–not evaluable).
Results
Factors associated with tafamidis prescription in a multivariable Cox proportional hazards regression (hazard ratio [95% CI]) included age ≥ 65 years (2.1 [1.07–4.05]), male sex (1.6 [1.07–2.28]), having heart failure/cardiomyopathy (2.4 [1.54–3.82]), and having had technetium-99m pyrophosphate myocardial scintigraphy (1.7 [1.28–2.28]).
Conclusions
The clinical characteristics of patients with ATTRwt-CM who were prescribed tafamidis in the real world were broadly comparable with those who took part in ATTR-ACT. Further studies are needed to evaluate hereditary and ATTRwt-CM patient populations in the real world and assess the long-term outcomes associated with disease management pathways.
Clinical Trials Registration
ClinicalTrials.gov identifier: NCT01994889.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
430 patients with transthyretin amyloid cardiomyopathy were identified in the Optum electronic health record database; 49% (n = 209) were prescribed tafamidis (median time to prescription: 5.8 months). |
Real-world patients prescribed tafamidis were broadly similar to patients in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT). |
We identified several factors associated with tafamidis prescription: older age, male sex, cardiac comorbidities, and technetium-99m pyrophosphate scintigraphy. |
1 Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive and usually fatal condition arising from the deposition of amyloid transthyretin (ATTR) in the myocardium [1, 2]. Hereditary transthyretin amyloid cardiomyopathy (ATTRv-CM) occurs due to a genetic variant in the TTR gene that promotes amyloid formation. ATTR formation can also be idiopathic and associated with aging, which can cause a condition called wild-type ATTR-CM (ATTRwt-CM) [1, 2]. Without appropriate management and treatment, survival for patients with ATTRwt-CM is reported to be approximately 3.6 years; however, this may be increased with earlier diagnosis and treatment [3,4,5,6,7,8,9]. Evidence suggests that ATTRwt-CM is an underdiagnosed cause of heart failure in older adults [7, 8, 10,11,12]. Missed or delayed diagnosis of ATTRwt-CM can be frequent, further delaying access to disease-modifying treatment [8, 9, 11].
Until recently, no pharmacologic treatment was indicated for patients with ATTR-CM, potentially disincentivizing the pursuit of early diagnosis. Tafamidis was approved by the United States Food and Drug Administration (US FDA) in May 2019 as the first disease-modifying treatment for ATTR-CM [13]. This was based on data from the phase III Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT), where tafamidis was found to reduce all-cause mortality and cardiovascular-related hospitalizations after 30 months of treatment in patients with hereditary or wild-type ATTR-CM compared with placebo [14]. The recent multi-societal clinical practice guidelines recommend tafamidis in select patients with ATTR-CM, but as a relatively new therapy, there are limited real-world data on the use of tafamidis in clinical practice [15].
The aim of this analysis was to summarize the characteristics of patients with ATTRwt-CM who have and have not been prescribed tafamidis in the US by using real-world evidence from Optum de-identified electronic healthcare records (EHRs). These real-world findings may help to further contextualize the ATTR-ACT population.
2 Methods
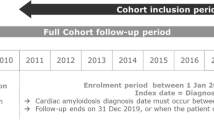
This real-world, retrospective, observational cohort study included patients with ATTRwt-CM in the US. Patients were indexed from Optum’s de-identified, longitudinal EHR database between 3 May 2019 (FDA approval of tafamidis) and 31 December 2020, at diagnosis of ATTRwt-CM (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code E85.82) or prescription of tafamidis (or both). Optum’s longitudinal EHR repository includes > 700 hospitals and > 7000 clinics, treating > 104 million patients receiving care in the US. De-identified Optum data from physician offices, emergency departments, laboratories, and hospitals include demographics, medications prescribed, laboratory results, vital signs, clinical and inpatient stay administrative data, and coded diagnoses and procedures.
Inclusion criteria for this analysis were a documented diagnosis of ATTRwt-CM (ICD-10-CM code E85.82 or tafamidis prescription), age ≥ 18 years, and ≥ 6 months’ activity in the database prior to index. In the US, tafamidis is only approved for the treatment of ATTR-CM, therefore prescription assumes an underlying diagnosis. Patients with a diagnosis code for light chain amyloidosis (ICD-10-CM: E85.81) in the 6 months before index were excluded, per recommendations for the diagnosis of ATTR-CM [16]. Patients were followed until first tafamidis prescription, or in the case of no prescription, until death or last activity in the database (whichever occurred first).
The primary objective of this analysis was to describe the baseline characteristics of patients with ATTRwt-CM who were and were not prescribed tafamidis at any time from index to end of follow-up (inclusive). The secondary objective was to identify baseline characteristics associated with the likelihood of tafamidis prescription in patients with ATTRwt-CM. Furthermore, we looked at the previously published characteristics of patients enrolled in ATTR-ACT in the context of our findings for patients with ATTRwt-CM in the real world.
Descriptive statistics were calculated for all baseline characteristics and presented by group (prescribed tafamidis vs. not prescribed tafamidis) using mean and standard deviation for continuous variables and counts and percentages for categorical or ordinal variables. Diagnoses were coded using the ICD-10-CM; procedures by Current Procedural Terminology, Fourth Edition, Healthcare Common Procedure Coding System, and ICD-10 Procedural Classification System; and prescription drugs by the National Drug Code. The baseline period was defined as the 6 months prior to index to ensure applicable data were captured. Comorbidities of interest were identified for their role in the etiology of amyloidosis, as indicators of heart failure, or their relationship as ‘red flags’ for ATTRwt-CM (electronic supplementary material [ESM] Table 1) [7, 8, 11, 17,18,19]. The length of time from index to first tafamidis prescription was analyzed as a time-to-event variable and summarized using the Kaplan–Meier method. Patients indexed on tafamidis prescription were entered at time zero. The independent association between baseline characteristics and the likelihood of tafamidis prescription were assessed for all patients using Cox proportional hazards regression. Characteristics that were significant (p < 0.05) in the univariable model were included in the final multivariable model. There was no imputation for missing data. Covariate-adjusted hazard ratio (HR) with two-sided 95% confidence interval (CI) is reported for each variable in the model. All p-values are two-sided. No adjustments for multiplicity were considered since this was a retrospective analysis [20]. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 Results
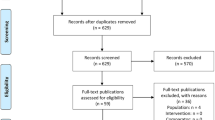
Of the 37,724,217 patients in the US Optum EHR database between May 2019 and December 2020, 430 patients with ATTRwt-CM (0.001%) met the criteria for inclusion in this study (Fig. 1). Overall, 72% (n = 311/430) of patients were indexed without a tafamidis prescription, whereas 28% were indexed with a tafamidis prescription. The median duration from index to first tafamidis prescription, or in the case of no prescription, death or last activity in the database, for all 430 patients was 1.5 months (25th percentile: 1 day; 75th percentile: 7 months). At the end of follow-up, 49% of patients had been prescribed tafamidis (21% more than at index), whereas 51% were diagnosed with ATTRwt-CM at index and were never prescribed tafamidis.
Baseline characteristics for the 430 patients who were (n = 209) or were not (n = 221) prescribed tafamidis during follow-up are shown in Table 1. Patients prescribed versus those never prescribed tafamidis were, on average, slightly older (mean age: 78 vs. 72 years), more likely to be male (85% vs. 71%), and more likely to be White (75% vs. 70%). More than half of the patients in each cohort had Medicare or Medicaid insurance and had a cardiologist associated with the team providing the index ATTRwt-CM diagnosis. The method associated with index ATTRwt-CM diagnosis was not recorded for 45% of patients (43% of patients who were prescribed tafamidis and 66% of those who were not). Among those with a recorded diagnostic method, technetium-99m pyrophosphate (99mTc-PYP) myocardial scintigraphy using single photon emission computed tomography was associated with the index ATTRwt-CM diagnosis for 68% (n = 81/120) of patients who were prescribed tafamidis and 44% (n = 33/75) of patients who were not prescribed tafamidis.
Data on New York Heart Association (NYHA) class, left ventricular ejection fraction (LVEF), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration from the previous 6 months were missing for a large proportion of patients, likely because these data are not routinely documented in discrete fields within the EHRs. Of patients with reported values, the majority were NYHA class II (48% [n = 30/63] of patients prescribed tafamidis and 32% [n = 8/25] of those not prescribed tafamidis) or class III (49% [n = 31/63] and 64% [n = 16/25], respectively). An LVEF ≥50% at baseline was noted for 49% (n = 62/127) of patients who were prescribed tafamidis and 54% (n = 51/94) of patients who were not (ESM Table 2). Median NT-proBNP concentration was 2911 pg/mL (interquartile range [IQR] 3567 pg/mL; n = 75) for patients prescribed tafamidis and 1644 pg/mL (IQR 3542 pg/mL; n = 51) for those who were not prescribed tafamidis.
During the baseline period, several of the comorbidities of interest were found in a greater proportion of patients who were prescribed tafamidis than among those who were not prescribed tafamidis (Table 2). Overall, 89% of patients who were prescribed tafamidis had comorbidities of heart failure or cardiomyopathy compared with 62% of those who were not prescribed tafamidis. Similarly, a higher proportion of patients prescribed tafamidis had cardiac arrhythmias or conduction disorders (71% vs. 53%). Non-cardiovascular comorbidities had comparatively lower incidences, and differences between the cohorts were not as pronounced.
In the 6 months pre index, few patients were prescribed diflunisal (1% [n = 3] of those prescribed tafamidis during follow-up vs. 1% [n = 3] of those not prescribed tafamidis) or ATTR silencers (0% vs. 4% [n = 8]). Most cardiovascular medications of interest were prescribed to a higher proportion of patients who were prescribed tafamidis during follow-up than to those who were not prescribed tafamidis (ESM Table 3).
Overall, 61% of patients who received a tafamidis prescription first received it from a team that included a cardiologist during the healthcare encounter (Table 3). All patients who were prescribed tafamidis during follow-up were within 9 months of index (including those prescribed tafamidis at index [time = 0]). The majority (80%) were prescribed tafamidis within 1 month of index (Table 3). The median time to tafamidis prescription was 5.8 months from index (95% CI 2.43–not evaluable) (Fig. 2).
Univariable analyses showed a significantly higher likelihood of tafamidis prescription in patients who were aged ≥ 65 years and male, with heart failure or cardiomyopathy, cardiac arrhythmias or conduction disorders, and Medicare/Medicaid insurance; had been indexed during a visit to a cardiologist; or had undergone 99mTc-PYP myocardial scintigraphy (ESM Fig. 1). Factors associated with an increased likelihood of tafamidis prescription in the multivariable analysis (HR [95% CI]) were age ≥ 65 years (2.08 [1.07–4.05]), male sex (1.56 [1.07–2.28]), heart failure or cardiomyopathy (2.42 [1.54–3.82]), and 99mTc-PYP myocardial scintigraphy (1.71 [1.28–2.28]) (Fig. 3). Other or unknown medical insurance was also associated with a significantly increased likelihood of tafamidis prescription compared with commercial insurance. As only 11 patients who were prescribed tafamidis were aged < 65 years, and 68 patients prescribed tafamidis had unknown insurance, it is likely that the majority of patients prescribed tafamidis with missing insurance status were aged ≥ 65 years and Medicare-insured.
When reviewing the characteristics of patients with ATTRwt-CM in ATTR-ACT [14] and the current real-world study, the proportion who were male and White appeared to be slightly higher in ATTR-ACT (ESM Table 4). In addtion, ATTR-ACT was an international study, whereas this real-world analysis only included patients from the US. Although much of the data on cardiac function were missing from the records, around one-quarter of patients with ATTRwt-CM and treated with tafamidis in ATTR-ACT were NYHA class III at baseline versus almost half of those in this study. Mean NT-proBNP concentration appeared to be higher in patients in the real-world analysis than in ATTR-ACT. Among placebo-treated patients in ATTR-ACT, mean LVEF appeared higher, and the proportion with comorbidities of hypertension and diabetes lower, than in patients with ATTRwt-CM in the real world.
4 Discussion
This study of real-world patients with ATTRwt-CM showed that those who were prescribed tafamidis were broadly similar but in marginally poorer clinical condition than those who were not prescribed tafamidis. Multivariable analysis showed age ≥ 65 years, male sex, having heart failure or cardiomyopathy, and having undergone 99mTc-PYP myocardial scintigraphy were significant predictors for tafamidis prescription. Many variables collected as part of randomized controlled trials were not commonly documented in real-world clinical practice. Despite this, our overall results indicated the characteristics of people in the US who were prescribed tafamidis for the treatment of ATTRwt-CM between May 2019 and December 2020 appeared broadly comparable with those enrolled in ATTR-ACT [3, 14, 21]. Our findings show that many patients with ATTRwt-CM were not prescribed tafamidis, suggesting an unmet treatment need. An increased awareness of treatment patterns can help identify undertreated populations and treatment barriers.
As current real-world evidence on the use of tafamidis is lacking, this study provides unique insight into the continued underdiagnosis and undertreatment of ATTRwt-CM. Although considered a rare condition, we suspect that the 0.001% of the Optum EHR population diagnosed with ATTRwt-CM or treated with tafamidis between May 2019 and December 2020 is likely to be an underestimate of the affected population, and potentially capturing more severely affected patients. This may reflect the type of records held in the Optum database and the low clinical awareness and suspicion of the disease during that time [7, 8, 10, 11]. Although the true prevalence of ATTRwt-CM is unknown, autopsy findings have found wild-type ATTR in 17% of people with heart failure with preserved ejection fraction (HFpEF) and 5% of people without [22]. Similarly, in a Spanish clinical setting, 13% of patients aged ≥ 60 years with HFpEF were highly likely to have ATTRwt-CM [10]. Another study found ATTR in 25% of people aged ≥ 85 years, irrespective of previously reported symptoms [23]. Diagnoses in EHR data can only be reliably inferred from ICD codes. While there is no code for ATTRv amyloidosis, the code for ATTRwt amyloidosis was introduced in October 2017. Tafamidis was approved for prescription in the US in May 2019. Together, these methods were used to identify patients with ATTRwt-CM in our study; however, both were relatively new during the study period and this may have resulted in some affected patients not being indexed. Further education of clinicians around the ‘red flags’ for ATTR-CM and the availability of disease-modifying therapy may improve diagnosis and patients’ access to treatment.
We found that approximately half of all patients with ATTRwt-CM were prescribed tafamidis, with a median time to prescription of 6 months from index (80% within 1 month). Initial tafamidis prescriptions were most commonly made by a team that included a cardiologist. The prescribers for these patients could be considered ‘early adopters’, but any delay in obtaining guideline-recommended management for this progressive condition might reasonably limit the ability of patients to achieve optimal survival. The high proportion of patients not prescribed tafamidis suggests that treatment barriers exist. Little has been published on these barriers; they may include factors such as access, insurance coverage, cost, delayed or misdiagnosis, and lack of awareness of disease-modifying therapy [4, 9, 24]. Further study to identify these barriers is warranted.
Baseline factors significantly associated with the likelihood of tafamidis prescription in our multivariable analysis were age ≥ 65 years, male sex, heart failure or cardiomyopathy, and having undergone 99mTc-PYP myocardial scintigraphy. Patients outside these groups may experience barriers to treatment that should be investigated. Recent heart failure management guidelines recommend further evaluation of patients with clinical suspicion for ATTR-CM [15]. 99mTc-PYP myocardial scintigraphy is most commonly used to diagnose ATTR-CM because it is noninvasive, accessible, and has high sensitivity/specificity when used alongside testing for free monoclonal light chains [8, 17,18,19, 25, 26]. In our study, other or unknown medical insurance was also associated with a significantly increased likelihood of tafamidis prescription compared with commercial insurance. Although the insurance status of these patients is unknown, based on their age, we would suggest that these were largely Medicare-insured patients.
Misdiagnosis and patients with early disease (and possibly unreported or limited symptoms) may account for undiagnosed cases [7, 8, 10,11,12]. An increasing number of publications have aimed to delineate the clinical clues that should raise suspicion of ATTR-CM [7, 8, 11, 17,18,19, 27, 28]. These red flags typically include thickening of the left ventricular wall due to amyloidosis, HFpEF, elevated cardiac biomarkers, conduction disorder, atrial arrhythmias, pericardial effusion, polyneuropathy, carpal tunnel syndrome, lumbar spinal stenosis, degenerative joint disease, and atraumatic tendon rupture [7, 8, 11, 17,18,19, 27, 28]. Expert consensus recommendations suggest that a diagnosis of ATTR-CM should be considered in patients with signs of heart failure and one or more of these red flags [7, 8, 11, 15, 17,18,19, 27, 28]. Research and predictive analytics are underway to create screening and diagnostic clinical decision support tools for ATTR-CM based on these red flags [2, 11, 27,28,29]. These initiatives may improve the speed and accuracy of ATTR-CM diagnosis.
4.1 Limitations
Although the Optum EHR database offers a rich source of high-quality real-world data, the main limitations of this study are common to analyses of retrospective data. The Optum database contains EHRs from commercially insured patients. Other EHR databases may reveal a higher incidence of ATTRwt-CM, for example, among Medicare-insured patients. Common to EHR analyses, causative associations cannot be confirmed. Furthermore, we acknowledge that coding errors do occur in EHR data (e.g., incorrectly noting or not noting an ATTRwt-CM diagnosis, medication use, comorbidities or diagnostic modality). In our study, ICD coding and tafamidis prescription were used to identify patients with ATTRwt-CM. Tafamidis is also indicated for ATTRv-CM, and we did not aim to exclude the possibility that a small number of patients receiving tafamidis without an ATTRwt-CM diagnosis had the hereditary form, for which an ICD code is not available. Introduction of an ICD code for ATTRv-CM would permit further study. We utilized only a 6-month baseline period, and this may have led to some comorbidities not being captured, such as heart failure. A large degree of missingness was discovered for cardiac function and symptom measures, limiting our ability to compare heart failure status in the real-world population with patients in ATTR-ACT. Poor documentation of health failure status in real-world clinical practice may contribute to the lack of such data. For instance, only 19% of patients with heart failure had a recorded NYHA class in a major US academic health center EHR system. Among patients with information on NYHA, it was generally documented in unstructured fields of the EHR [30]. Additionally, dosing information was unclear due to different tafamidis formulations being available during the index period, and we cannot be sure that patients took their prescribed medication. Lastly, it is possible that some diagnoses and medication use took place outside of the Optum EHR database.
5 Conclusion
This study was the first to use large-scale, national, real-world EHR data to evaluate the demographics and clinical characteristics of patients prescribed tafamidis. We found broadly similar characteristics to those of patients who took part in the phase III ATTR-ACT. The median time from index to tafamidis prescription in the real world was 6 months. Of patients who were prescribed tafamidis, 80% were first prescribed within 1 month of index. Patients who were prescribed tafamidis were older, more likely to be male and to have heart failure or cardiomyopathy, and to have undergone 99mTc-PYP myocardial scintigraphy than those who were not prescribed tafamidis. Overall, those who were prescribed tafamidis appeared to have more advanced or symptomatic disease, which may reflect the need for earlier awareness in order to pursue diagnosis and treatment of ATTRwt-CM. Further work is needed to understand how the diagnostic journey for patients with ATTRwt-CM can be more efficient, allowing earlier access to applicable management plans and the survival benefit of treatment.
References
Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR amyloidosis: current and emerging management strategies: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2021;3:488–505. https://doi.org/10.1016/j.jaccao.2021.06.006.
Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–91. https://doi.org/10.1016/j.jacc.2019.04.003.
Nativi-Nicolau J, Judge DP, Hoffman JE, Gundapaneni B, Keohane D, Sultan MB, et al. Natural history and progression of transthyretin amyloid cardiomyopathy: insights from ATTR-ACT. ESC Heart Fail. 2021;8:3875–84. https://doi.org/10.1002/ehf2.13541.
Rozenbaum MH, Large S, Bhambri R, Stewart M, Young R, van Doornewaard A, et al. Estimating the health benefits of timely diagnosis and treatment of transthyretin amyloid cardiomyopathy. J Comp Eff Res. 2021;10:927–38. https://doi.org/10.2217/cer-2021-0071.
Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164:222-8.e1. https://doi.org/10.1016/j.ahj.2012.04.015.
Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–20. https://doi.org/10.1016/j.jacc.2016.06.033.
Witteles RM, Bokhari S, Damy T, Elliott PM, Falk RH, Fine NM, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7:709–16. https://doi.org/10.1016/j.jchf.2019.04.010.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12:e006075. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006075.
Rozenbaum MH, Large S, Bhambri R, Stewart M, Whelan J, van Doornewaard A, et al. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): a targeted literature review. Cardiol Ther. 2021;10:141–59. https://doi.org/10.1007/s40119-021-00219-5.
González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94. https://doi.org/10.1093/eurheartj/ehv338.
Gertz M, Adams D, Ando Y, Beirão JM, Bokhari S, Coelho T, et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Fam Pract. 2020;21:198. https://doi.org/10.1186/s12875-020-01252-4.
Bennani Smires Y, Victor G, Ribes D, Berry M, Cognet T, Méjean S, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32:1403–13. https://doi.org/10.1007/s10554-016-0915-z.
Pfizer. Prescribing information for VYNDAQEL (tafamidis meglumine capsule, liquid filled) and VYNDAMAX (tafamidis capsule, liquid filled). 2019. http://labeling.pfizer.com/ShowLabeling.aspx?id=11685. Accessed 31 Mar 2022.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–16. https://doi.org/10.1056/NEJMoa1805689.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e876–94. https://doi.org/10.1161/CIR.0000000000001062.
Hafeez AS, Bavry AA. Diagnosis of transthyretin amyloid cardiomyopathy. Cardiol Ther. 2020;9:85–95. https://doi.org/10.1007/s40119-020-00169-4.
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2–evidence base and standardized methods of imaging. J Cardiac Fail. 2019;25:e1-39. https://doi.org/10.1016/j.cardfail.2019.08.001.
Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2–diagnostic criteria and appropriate utilization. J Nuclear Cardiol. 2020;27:659–73. https://doi.org/10.1007/s12350-019-01761-5.
Rigopoulos AG, Ali M, Abate E, Torky AR, Matiakis M, Mammadov M, et al. Advances in the diagnosis and treatment of transthyretin amyloidosis with cardiac involvement. Heart Fail Rev. 2019;24:521–33. https://doi.org/10.1007/s10741-019-09776-3.
Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6.
Rapezzi C, Elliott P, Damy T, Nativi-Nicolau J, Berk JL, Velazquez EJ, et al. Efficacy of tafamidis in patients with hereditary and wild-type transthyretin amyloid cardiomyopathy: further analyses from ATTR-ACT. JACC Heart Fail. 2021;9:115–23. https://doi.org/10.1016/j.jchf.2020.09.011.
Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–22. https://doi.org/10.1016/j.jchf.2013.11.004.
Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–9. https://doi.org/10.1080/07853890701842988.
Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141:1214–24. https://doi.org/10.1161/CIRCULATIONAHA.119.045093.
Nguyen AT, Alexander KM. Mistaken identity: using bone scintigraphy to diagnose cardiac amyloidosis in patients with a monoclonal gammopathy. JACC CardioOncol. 2021;3:594–7. https://doi.org/10.1016/j.jaccao.2021.06.002.
Bourque JM, Schepart A, Bhambri R, Castaño A, O’Brien A, Chen Y, et al. Temporal trends in diagnostic testing patterns for wild-type transthyretin amyloid cardiomyopathy in the Medicare fee-for-service population. Am J Cardiol. 2022;167:98–103. https://doi.org/10.1016/j.amjcard.2021.11.048.
Huda A, Shah SJ, Castano A, Niyogi A, Schumacher J, Stewart M, et al. A machine learning model for the systematic identification of wild-type transthyretin cardiomyopathy. J Cardiac Fail. 2019;25:S53–4. https://doi.org/10.1016/j.cardfail.2019.07.151.
Huda A, Castaño A, Niyogi A, Schumacher J, Stewart M, Bruno M, et al. A machine learning model for identifying patients at risk for wild-type transthyretin amyloid cardiomyopathy. Nat Commun. 2021;12:2725. https://doi.org/10.1038/s41467-021-22876-9.
Willis C, Kawamoto K, Watanabe A, Biskupiak J, Nolen K, Blackner L, et al. Screening of patients at risk for wild-type ATTR-CM using a computational machine learning algorithm. Can J Cardiol. 2021;37:S65. https://doi.org/10.1016/j.cjca.2021.07.134.
Zhang R, Ma S, Shanahan L, Munroe J, Horn S, Speedie S. Discovering and identifying New York heart association classification from electronic health records. BMC Med Inform Decis Mak. 2018;18:48. https://doi.org/10.1186/s12911-018-0625-7.
Acknowledgments
Medical writing support (drafting and collating author feedback) was provided by Jennifer Bodkin of Engage Scientific Solutions and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Pfizer.
Role of the Funding Source
Pfizer contributed to the study design and management and collection of data. In their role as authors, employees of Pfizer were involved in the interpretation of data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication, along with their co-authors. The study sponsor approved the manuscript from an intellectual property perspective but had no right to veto the publication.
Conflicts of Interest/Competing Interests
Rahul Bhambri, A. Carmine Colavecchia, Marianna Bruno, Yong Chen, Jose Alvir, Anuja Roy, and Jason Kemner are employees of Pfizer and own stock/stock options. Aaron Crowley and Darrin Benjumea are employees of Genesis Research, LLC, which was contracted and paid by Pfizer for participation in this study. Lauren Gilstrap has no relevant disclosures.
Ethics Approval
This study was conducted in accordance with the Code of Ethics of the World Medical Association (the Declaration of Helsinki).
Consent to participate/Consent for publication
This study was based on de-identified data from Optum’s longitudinal EHR. No additional consent was required to conduct or publish this analysis.
Availability of Data and Material
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Code availability
Comorbidity diagnostic codes are provided in the Supplementary Material.
Author Contributions
RB, ACC, MB, YC, JA, AR, JK, AC, and DB contributed to the study conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, and writing – review and editing. RB, ACC, MB, YC, JA, AR, and JK additionally contributed to the funding acquisition and resourcing. LG contributed to the formal analysis, investigation, validation, visualization, and writing – review and editing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bhambri, R., Colavecchia, A.C., Bruno, M. et al. Real-World Characteristics of Patients with Wild-Type Transthyretin Amyloid Cardiomyopathy: An Analysis of Electronic Healthcare Records in the United States. Am J Cardiovasc Drugs 23, 197–206 (2023). https://doi.org/10.1007/s40256-022-00563-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00563-4