Abstract
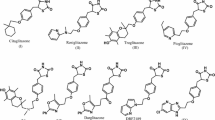
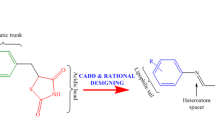
The finest sources of therapeutic agents are natural products, and usnic acid is a secondary metabolite derived from lichen that has a wide range of biological actions, including anti-viral, anti-cancer, anti-bacterial, and anti-diabetic (hyperglycemia). Based on the hyperglycemia activity of UA, this work seeks to identify new anti-hyperglycemia medicines by virtual screening of pyrazole derivatives of UA. Seven hit compounds (Compounds 1, 5, 6, 7, 17, 18 and 33), which finally go through docking-based screening to produce the lead molecule, were identified by the physicochemical attributes, drug-likeliness, and ADMET prediction. The docking score for the chosen compounds containing PPARγ agonists ranged from -7.6 to -9.2 kcal/mol, whereas the docking goal for compounds 5, 6, and 7 was -9.2 kcal/mol. Based on the binding energy and bound amino acid residues as well as compared to the reference compound, compound-6 considered as lead compound. Furthermore, the MD simulation of 3CS8-Compound-6 and 3CS8-Rosiglitazone complexes were performed to verify the stability of these complexes and the binding posture acquired in docking experiments. The compound-6 had strong pharmacological characteristics, bound to the PPARγ agonist active site, and was expected to reduce the activity of the receptor, according to the virtual screening results. It must be justified to conduct both in-vitro and in-vivo experiments to examine the efficacy of this compound.









Similar content being viewed by others
References
Abdelgawad MA, El-Adl K, El-Hddad SS, Elhady MM, Saleh NM, Khalifa MM, Abd El-Sattar NE (2022) Design, molecular docking, synthesis, anticancer and anti-hyperglycemic assessments of thiazolidine-2, 4-diones bearing Sulfonylthiourea moieties as potent VEGFR-2 inhibitors and PPARγ. Agonists Pharmaceuticals 15(2):226
Alam MJ, Alam O, Alam P, Naim MJ (2015) A review on pyrazole chemical entity and biological activity. Int J Pharm Sci Res 6:1433–1442
Amin RP, Patel SN, Kumar S, Zito WS, Barletta MA (2018) Effects of usnic acid on hyperglycemia and renal function in streptozotocin-induced diabetic rats. Arc Nat Med Chem 2018 (2):1–8
Araújo HDAD, Silva HAMF, Silva Júnior JGD, Albuquerque MCPDA, Coelho LCBB, Aires ADL (2021) The natural compound hydrophobic usnic acid and hydrophilic potassium usnate derivative: applications and comparisons. Mol 26(19):5995
Behera BC, Mahadik N, Morey M (2012) Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm Biol 50(8):968–979
Borisov SA, Luzina OA, Khvostov MV, Tolstikova TG, Salakhutdinov NF (2022) Synthesis and Pharmacological Evaluation of (+)-Usnic Acid Derivatives as Hypoglycemic Agents. Molbank 2022 (4):M1459
Brown JD, Plutzky J (2007) Peroxisome proliferator–activated receptors as transcriptional nodal points and therapeutic targets. Circulation 115(4):518–533
Cocchietto M, Skert N, Nimis P, Sava G (2002) A review on usnic acid, an interesting natural compound. Naturwissenschaften 89:137–146
Dey D, Hossain R, Biswas P, Paul P, Islam MA, Ema TI, Kim B (2023) Amentoflavone derivatives significantly act towards the main protease (3CLPRO/MPRO) of SARS-CoV-2: in silico admet profiling, molecular docking, molecular dynamics simulation, network pharmacology. Mol Divers 27(2):857–871
Guo HY, Jin C, Zhang HM, Jin CM, Shen QK, Quan ZS (2019) Synthesis and biological evaluation of (+)-Usnic acid derivatives as potential anti-toxoplasma gondii agents. J Agric food chem 67(34):9630–9642
Guzow-Krzemińska B, Guzow K, Herman-Antosiewicz A (2019) Usnic acid derivatives as cytotoxic agents against cancer cells and the mechanisms of their activity. Curr Pharmacol Rep 5(6):429–439
Hernández-Vázquez E, Ocampo-Montalban H, Cerón-Romero L, Cruz M, Gómez-Zamudio J, Hiriart-Valencia G, Estrada-Soto S (2017) Antidiabetic, antidyslipidemic and toxicity profile of ENV-2: a potent pyrazole derivative against Diabetes and related Diseases. Eur J Pharmacol 803:159–166
Huang Z, Zheng G, Tao J, Ruan J (2011) Anti-inflammatory effects and mechanisms of usnic acid. J Wuhan Univ Technol-Mater Sci Ed 26:955–959
Ibrahim MK, Eissa IH, Abdallah AE, Metwaly AM, Radwan MM, ElSohly MA (2017) Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of novel quinoxaline derivatives as potential PPARγ and SUR agonists. Bioorg med chem 25(4):1496–1513
Janani C, Kumari BR (2015) PPAR gamma gene–a review. Diabetes Metab Syndr: Clin Res Rev 9(1):46–50
Jorgensen WL, Tirado-Rives J (1988) The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc 110(6):1657–1666
Kiliç N, Islakoğlu YÖ, Büyük İ, Gür-Dedeoğlu B, Cansaran-Duman D (2019) Determination of usnic acid responsive miRNAs in Breast cancer cell lines. Anti-Cancer Agents Med Chem 19(12):1463–1472
Koparal AT, Ayaz Tüylü B, Türk H (2006) In vitro cytotoxic activities of (+)-usnic acid and (–)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat Prod Res 20(14):1300–1307
Langheinrich U, Vacun G, Wagner T (2003) Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging Drugs inducing severe arrhythmia☆. Toxicol appl Pharmacol 193(3):370–382
Li Y, Kovach A, Suino-Powell K, Martynowski D, Xu HE (2008) Structural and biochemical basis for the binding selectivity of peroxisome proliferator-activated receptor γ to PGC-1α. J Biol Chem 283(27):19132–19139
Lingineni K, Belekar V, Tangadpalliwar SR, Garg P (2017) The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood–brain barrier (BBB) permeability. Mol Divers 21:355–365
Liu Y, Grimm M, Dai WT, Hou MC, Xiao ZX, Cao Y (2020) CB-Dock: a web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol Sin 41(1):138–144
Luzina OA, Salakhutdinov NF (2016) Biological activity of usnic acid and its derivatives: part 1. Activity against unicellular organisms. Russ J Bioorganic Chem 42(2):115–132
Mallavadhani UV, Vanga NR, Rao KB, Jain N (2019) Synthesis and antiproliferative activity of novel (+)-usnic acid analogues. J Asian nat prod res
Mayer M, O’Neill MA, Murray KE, Santos-Magalhães NS, Carneiro-Leão AMA, Thompson AM, Appleyard VC (2005) Usnic acid: a non-genotoxic compound with anti-cancer properties. Anticancer Drugs 16(8):805–809
Nagar PR, Gajjar ND, Dhameliya TM (2021) In search of SARS CoV-2 replication inhibitors: virtual screening, molecular dynamics simulations and ADMET analysis. J Mol Struct 1246:131190
Naim MJ, Alam O, Alam MJ, Hassan MQ, Siddiqui N, Naidu VGM, Alam MI (2018) Design, synthesis and molecular docking of thiazolidinedione based benzene sulphonamide derivatives containing pyrazole core as potential anti-diabetic agents. Bioorg chem 76:98–112
Newman DJ, Cragg GM (2016) Natural products as sources of new Drugs from 1981 to 2014. J nat prod 79(3):629–661
Nishinarizki V, Hardianto A, Gaffar S, Muchtaridi M, Herlina T (2023) Virtual screening campaigns and ADMET evaluation to unlock the potency of flavonoids from Erythrina as 3CLpro SARS-COV-2 inhibitors. J Appl Pharm Sci 13(2):078–088
Patil SB (2020) Medicinal siganificance of pyrazole analogues: a review. J Pharm Sci Res 12(3):402–404
Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T (2009) Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers med sci 24:259–268
Pogaku V, Gangarapu K, Basavoju S, Tatapudi KK, Katragadda SB (2019) Design, synthesis, molecular modelling, ADME prediction and anti-hyperglycemic evaluation of new pyrazole-triazolopyrimidine hybrids as potent α-glucosidase inhibitors. Bioorg chem 93:103307
Pyrczak-Felczykowska A, Narlawar R, Pawlik A, Guzow-Krzemińska B, Artymiuk D, Hać A, Kassiou M (2019) Synthesis of usnic acid derivatives and evaluation of their antiproliferative activity against cancer cells. J nat prod 82(7):1768–1778
Rakhmanova ME, Luzina OA, Pokrovskii MA, Pokrovskii AG, Salakhutdinov NF (2016) Synthesis and cytotoxic activity of usnic acid cyanoethyl derivatives. Russ Chem Bull 65(2):566–569
Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Aminabhavi TM (2005) Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s Disease. J Controlled Release 108(2–3):193–214
Roney M, Huq AM, Rullah K, Hamid HA, Imran S, Islam MA, Mohd Aluwi MFF (2021) Virtual screening-based identification of potent DENV-3 RdRp protease inhibitors via in-house usnic acid derivative database. J Comput Biophys Chem 20(08):797–814
Roney M, Huq AM, Issahaku AR, Soliman ME, Hossain MS, Mustafa AH, Tajuddin SN (2023) Pharmacophore-based virtual screening and in-silico study of natural products as potential DENV-2 RdRp inhibitors. J Biomol Struct Dyn 1–18
Saeed M, Shoaib A, Tasleem M, Alabdallah NM, Alam MJ, Asmar ZE, Badraoui R (2021) Assessment of antidiabetic activity of the shikonin by allosteric inhibition of protein-tyrosine phosphatase 1B (PTP1B) using state of art: an in silico and in vitro tactics. Mol 26(13):3996
Shi CJ, Peng W, Zhao JH, Yang HL, Qu LL, Wang C, Wang XB (2020) Usnic acid derivatives as tau-aggregation and neuroinflammation inhibitors. Eur j med chem 187:111961
Shtro AA, Zarubaev VV, Luzina OA, Sokolov DN, Kiselev OI, Salakhutdinov NF (2014) Novel derivatives of usnic acid effectively inhibiting reproduction of Influenza a virus. Bioorg med chem 22(24):6826–6836
Shtro AA, Zarubaev VV, Luzina OA, Sokolov DN, Salakhutdinov NF (2015) Derivatives of usnic acid inhibit broad range of Influenza viruses and protect mice from lethal Influenza Infection. Antivir Chem Chemother 24(3–4):92–98
Zakharenko AL, Luzina OA, Sokolov DN, Zakharova OD, Rakhmanova ME, Chepanova AA, Salakhutdinov NF (2017) Usnic acid derivatives are effective inhibitors of tyrosyl-DNA phosphodiesterase 1. Russ J Bioorg Chem 43(1):84–90
Acknowledgements
The authors would like to thank the Ministry of Higher Education for providing financial support under the Fundamental Research Grant Scheme (FRGS) No. FRGS/1/2019/STG01/UMP/02/4 (University Reference: RDU1901160) and Universiti Malaysia Pahang Al-Sultan Abdullah for providing lab facilities. Furthermore, the authors would like to thanks to the online software teams.
Author information
Authors and Affiliations
Contributions
M.R. and A. R. I. conducted the experiments, results analysis and drafted the manuscript. M.F.F.M.A. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roney, M., Issahaku, A.R. & Aluwi, M.F.F.M. Virtual screening of pyrazole derivatives of usnic acid as new class of anti-hyperglycemic agents against PPARγ agonists. In Silico Pharmacol. 11, 36 (2023). https://doi.org/10.1007/s40203-023-00176-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-023-00176-y