Abstract
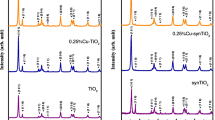
Natural organic matters are of particular importance in drinking water treatment due to their reaction with chlorine, and formation of disinfection byproducts that cause cancer in humans. Photocatalysis can remove natural organic matters from water but usually powdery photocatalysts are used which should be separated from water by filtration due to their toxic effects. In this work, a piece of copper cable used in electric industries was doped with tin oxide and applied as a photocatalyst to remove natural organic matters, humic acid and humate liquid fertilizer, from water. Tin (II) chloride was used as precursor, and deposited on the copper cable by dip coating method. Then the coated cable was calcinated at 300 °C. The prepared SnO2/CuO/Cu photocatalyst was characterized by ICP, SEM, DRS, XRD, and ASAP techniques. The results of XRD confirmed the existence of copper oxide, and tin oxides. DRS showed that doping with tin oxide caused the photocatalytic property to improve, and the catalyst was active under irradiation of UV–Vis light. Effects of humic acid concentration, photocatalyst length, and time were studied. The kinetic of humic acid photodegradation by the SnO2/CuO/Cu photocatalyst was investigated, which obeyed the first order model. The photocatalyst regeneration and reuse were investigated in five cycles, and the results indicated that photocatalytic activity was remained nearly constant. The cable form SnO2/CuO/Cu photocatalyst with the main advantage of easy separation from water without the need to filtration, has excellent photocatalytic activity.











Similar content being viewed by others
References
Bhatnagar A, Sillanpää M. Removal of natural organic matter (NOM) and its constituents from water by adsorption e A review. Chemosphere. 2017;166:497–519.
Sillanpää M, Ncibi MC, Matilainen A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J Environ Manage. 2018;208:56–76.
Ndlangamandla NG, Kuvarega AT, Msagati TAM, Mamba BB, Nkambule TTI. A novel photodegradation approach for the efficient removal of natural organic matter (NOM) from water. Phys Chem Earth. 2018;106:97–106.
Gautam S, Agrawal H, Thakur M, Akbari A, Sharda H, Kaur R, Amini M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J Environ Chem Eng. 2020;8: 103726.
Kumar S, Ahlawat W, Bhanjana G, Heydarifard S, Nazhad MM, Dilbaghi N. Nanotechnology-based water treatment strategies. J Nanosci Nanotechnol. 2014;14:1838–58.
Gora S, Liang R, Zhou YN, Andrews S. Settleable engineered titanium dioxide nanomaterials for the removal of natural organic matter from drinking water. Chem Eng J. 2018;334:638–49.
Truong HB, Huy BT, Ly QV, Lee Y-I, Hur J. Visible light-activated degradation of natural organic matter (NOM) using zinc-bismuth oxides-graphitic carbon nitride (ZBO-CN) photocatalyst: Mechanistic insights from EEM-PARAFAC. Chemosphere. 2019;224:597–606.
Truong HB, Huy BT, Ray SK, Lee Y-I, Cho J, Hur J. H2O2-assisted photocatalysis for removal of natural organic matter using nanosheet C3N4-WO3 composite under visible light and the hybrid system with ultrafiltration. Chem Eng J. 2020;399: 125733.
Fattahi A, Arlos MJ, Bragg LM, Liang R, Zhou N, Servos MR. Degradation of natural organic matter using Ag-P25 photocatalyst under continuous and periodic irradiation of 405 and 365 nm UV-LEDs. J Environ Chem Eng. 2021;9: 104844.
Wieland S, Balmes A, Bender J, Kitzinger J, Meyer F. Ramsperger AFRM, Roeder F, Tengelmann C, Wimmer BH, Laforsch C, Kress H, From properties to toxicity: comparing microplastics to other airborne microparticles. J Hazard Mater. 2022;428: 128151.
Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12:908–31.
Jamuna BA, Ravishankar RV. Environmental risk, human health, and toxic effects of nanoparticles. In: Kharisov BI, Kharissova OV, Dias HVR, editors. Nanomaterials for environmental protection. John Wiley & Sons; 2014. p. 523–35.
Honda RJ, Keene V, Daniels L, Walker SL. Removal of TiO2 nanoparticles during primary water treatment: role of coagulant type, dose, and nanoparticle concentration. Environ Eng Sci. 2014;31:127–34.
Park CM, Chu KH, Her N, Jang M, Baalousha M, Heo J, Yoon Y. Occurrence and removal of engineered nanoparticles in drinking water treatment and wastewater treatment processes. Sep Purif Rev. 2017;46:255–72.
Heydari R, Akhlaghian F. Promotion of brass nanowires with lanthanum oxide and its application for photodegradation of tetracycline wastewater. Environ Sci Pollut Res. 2021;28:9255–66.
Mirzai M, Akhlaghian F, Rahmani F. Photodegradation of ciprofloxacin in water using photocatalyst of zinc oxide nanowires doped with copper and cerium oxides. Water Environ J. 2020;34:420–31.
Rajca M. The effectiveness of removal of nom from natural water using photocatalytic membrane reactors in PMR-UF and PMR-MF modes. Chem Eng J. 2016;305:169–75.
Ayekoe CYP, Robert D, Gone LD. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in realtreated water from Agbô River (Ivory-Coast). Catal Today. 2017;281:2–13.
Rao G, Jian X, Lv W, Zhu G, Xiong J, He W. A highly-efficient route to three-dimensional nanoporous copper leaves with high surface enhanced Raman scattering properties. Chem Eng J. 2017;321:394–400.
Fouda A, Salem SS, Wassel AR, Hamza MF, Shaheen TI. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon. 2020;6: e04896.
Fontaine-Gautrelet C, Thomas C, Djéga-Mariadassou G. Influence of the nature of the reducible support on CO oxidation kinetics of supported Rhδ+ catalysts: SnO2 versus Ce0.68Zr0.32O2. Top Catal. 2007;42–43:363–6.
Tada S, Ochieng OJ, Kikuchi R, Haneda T, Kameyama H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 Catalysts. Int J Hydrog Energy. 2014;39:10090–100.
Leofanti G, Padovan M, Tozzola G, Venturelli B. Surface area and pore texture of catalysts. Catal Today. 1998;41:207–19.
Mahmodi G, Sharifnia S, Rahimpour F, Hosseini SN. Photocatalytic conversion of CO2 and CH4 using ZnO coated mesh: Effect of operational parameters and optimization. Sol Energy Mater Sol Cells. 2013;111:31–40.
Xu Y, Schoonen MAA. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am Min. 2000;85:543–56.
Coulter JB, Birnie DB III. Assessing Tauc plot slope quantification: ZnO thin films as a model system. Phys Status Solidi B. 2018;255:1700393.
Ali MHH, Al-Qahtani KM, El-Sayed SM. Enhancing photodegradation of 2,4,6 trichlorophenol and organic pollutants in industrial effluents using nanocomposite of TiO2 doped with reduced graphene oxide. Egpt J Aquat Res. 2019;45:321–8.
Zhang X, Pan JH, Fu W, Du AJ, Sun DD. TiO2 nanotube photocatalytic oxidation for water treatment. Water Supply. 2009;9:45–9.
Parilti NB, Demirel CSU, Bekbolet M. Response surface methodological approach for the assessment of the photocatalytic degradation of NOM. J Photochem Photobiol A. 2011;225:26–35.
Oskoei V, Dehghani MH, Nazmara S, Heibati B, Asif M, Tyagi I, Agarwal S, Gupta VK. Removal of humic acid from aqueous solution using UV/ZnO nano-photocatalysis and adsorption. J Mol Liq. 2016;213:374–80.
Geng N, Chen W, Xu H, Ding M, Liu Z, Shen Z. A sono-photocatalyst for humic acid removal from water: Operational parameters, kinetics and mechanism. Ultrason Sonochem. 2019;57:242–52.
Khodadadi M, Al-Musawi TJ, Kamani H, Silva MF, Panahi AH. The practical utility of the synthesis FeNi3@SiO2@TiO2 magnetic nanoparticles as an efficient photocatalyst for the humic acid degradation. Chemosphere. 2020;239: 124723.
Elmougi M, El-Etriby H, Barakat R, Gar Alalm MG, Mossad M. Improving the ZnO-photocatalytic degradation of humic acid using powdered residuals from water purification plant. Water Pract Technol. 2022;17:1–13.
Acknowledgements
The financial support of the University of Kurdistan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamalvandi, P., Akhlaghian, F. Copper cable doped with tin oxide and its application to photodegrade natural organic matters. J Environ Health Sci Engineer 20, 555–563 (2022). https://doi.org/10.1007/s40201-022-00802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-022-00802-5