Abstract
Purpose
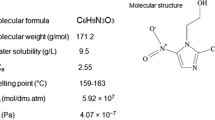
Pharmaceutical contaminants, including antibiotics, present in the environment, especially water resources, are a main concern for human and environmental health due to their stability and non-degradability. Accordingly, the purpose of this study was to investigate the photocatalytic removal of penicillin G antibiotic from simulated wastewater using a photocatalytic process [UV/ZnO] in an isotherm and kinetic study.
Methods
In the current research, the ZnO nanoparticles [ZnO NPs] were initially characterized by scanning electron microscope [SEM] and X-ray diffraction [XRD]. Then, its efficiency was investigated in the photocatalytic degradation process of penicillin G. The evaluated parameters in the adsorption process penicillin G antibiotic were pH [1,2,3,4,5], penicillin G concentration [10–30 mgL−1], NP dosage [0.5–4.5 gL−1] and contact time [5 to 200 min]. Then, the effect of pH [3, 5, 7, 9, 11, and], penicillin G concentration [10–30 mgL−1], NP dosage [0.01–1.5 gL−1] and contact time [5 to 200 min] in the photocatalytic degradation (UV/ZnO) was studied. The residual penicillin G concentration was measured using a spectrophotometery at a wavelength of 283 nm.
Results
The results indicated that the penicillin G removal efficiency of photocatalytic process [UV/ZnO] using ZnO was 74.65% at the concentration of 10 mgL−1, the pH value of 5, the ZnO NP dosage of 0.1 gL−1 and the contact time of 180 min, as well as the kinetics of degradation followed the pseudo-first-order kinetic model.
Conclusion
It can be concluded that the use of this process is appropriate an effective for the removal of the antibiotic pollutants.












Similar content being viewed by others
Change history
20 March 2020
The affiliation of the author Negin Nasseh was incorrect.
References
Emad SE, Chaudhuri M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination. 2010;256:43–7.
Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO photocatalysis. J Environ Manag. 2012;98:168–74.
María SL, Sandra S, Basaldellaab EI. Influence of pH on cephalexin adsorption onto SBA-15 mesoporous silica.
Carrasquillo AJ, Bruland GL, Mackay AA, et al. Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals: influence of compound structure. Environ Sci Technol. 2008;42(20):7643–2.
Watkinson A, Murby E, Costanzo S. Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res. 2007;41(18):4164–76.
Gagnon C, Lajeunesse A, Cejka P, Gagne F, Hausler R. Degradation of selected acidic and neutral pharmaceutical products in a primary-treated wastewater by disinfection processes. Ozone Sci Eng. 2008;30(5):387–92.
Dirany A, Sires I, Oturan N, Oturana MA. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere. 2010;81(5):594–602.
Elmolla S, Chaudhuri M. Comparison of different advanced oxidation processes for treatment antibiotic aqueos solution. Desalination. 2010;256:43–7.
Food and Drug Department. Ministry of Health. Pharmaceutical Statistics. 2003 to 2008;2009.
Levy SB. The antibiotic paradox, Da Capo Press, USA. 2002.
Valverde RS, Gil García MD, Galera MM, Goicoechea HC. Determination of tetracyclines in surface water by partial least squares using multivariate calibration transfer to correct the effect of solid phase preconcentration in photochemically induced fluorescence signals. Anal Chim Acta. 2006;562:85–93.
Yazdanbakhsh AR, Sheikhmohammadi A, Sardar M, Manshori M. Investigation of combined coagulation and advanced oxidationprocess efficiency for the removal of clarithromycin from wastewater. J Lorestan Univ Med Sci. 2011;13(1):7–16.
Vieno NM, Tuhkanen T. L. K. analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography-tandem mass spectrometry detection. J Chromatogr A. 2006;1134(1–2):101–11.
Le-Minh N, Khan SJ, Drewes JE, Stuetz RM. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010;44(15):4295–323.
Klavarioti M, Mantzavinos D, Kassinos D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int. 2009;35(2):402–17.
Isariebel QP, Carine JL, Ulises-Javier JH, Anne-Marie W, Henri D. Sonolysis ofle vodopa and paracetamol in aqueous solutions. Ultrason Sonochem. 2009;16(5):610–6.
Diasa IN, Souza BS, Pereira JH, Moreira FC, Dezottib M, Boaventura RA. Enhancement of the photo-Fenton reaction at near neutral pH through the use of ferrioxalate complexes: a case study on trimethoprim and sulfamethoxazole antibiotics removal from aqueous solutions. Chem Eng J. 2014;247:302–13.
Fukahori S, Fujiwara T. Modeling of sulfonamide antibiotic removal by TiO2/ high-silica zeolite HSZ-385 composite. J Hazard Mater. 2014;272:1–9.
Zazouli MA, Susanto H, Nasseri S, Ulbricht M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 2009;43(13):3270–2380.
Zazouli MA, Ulbricht M, Nasseri S, Susanto H. Effect of hydrophilic and hydrophobic organic matter on amoxicillin and cephalexin residuals rejection from water by nanofiltration. Iran J Environ Health Sci Eng 2010;7(1):15–24 (Persian).
Garoma T, Umamaheshwar SK, Mumper A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere. 2010;79(8):814–20.
Rahmani K, Rahmani A, Rahmani HR. Removal of tetracycline from aqueous solutions during the process of NanoZero Valent iron/UV/H2O2. J Environ Health Eng. 2013;4(2):295.
Mehralipour J, Leili M, Zolghadr Nasab H, Mohammadi AS. Efficiency of Electro /Fe2+/ Persulfate Process in Industrial Wastewater Treatment. J Mazandaran Univ Med Sci. 2015;25(123):137–48 (Persian).
Matilainen A, Sillanpaa M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere. 2010;80(4):351–65.
Adedapo R. “Disinfection by-product formation in drinking water treated with chlorine following UV photolysis and UV/H2O2.” In: Thesis Presented to the University of Waterloo O, Canada., editor (2005).
Busca G, Berardinelli S. Technologies for the removal of phenol from fluid stream: a short review of recent developments. J Hazard Mater. 2008;160:268–88.
Lucas MS, Peres JA, Puma GL. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep Purif Technol. 2010;72(3):235–41.
Rosenfeldt EJ, Linden KG, Canonica S, Gunten US. Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 2006;40(20):3695–704.
Nawrocki J, Kasprzyk-Hordern B. The efficiency and mechanisms of catalytic ozonation. Appl Catal B Environ. 2010;99(1):27–42.
Safari GH, Hosseini M, Komani H, Moradi Rad R, Hosseini M. Photocatalytic effect of antibiotic tetracycline from aqueous solutions using UV/TiO2/H2O2 and UV/TiO2. J Health Hygiene. 2014;5(3):203–13 [In persian].
Emad S, Chaudhuri M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater. 2010;173:445–9.
Shokri M, Hosseini, MGh, Najjar R, Yavari, MAA. The study of photocatalytic removal of penicillin antibiotics in aqueous solutions using TiO2 nanoparticles radiation uv. National Conference on Nano Sci Technol 2010:5.
Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices–a review. J Environ Manag. 2011;92(10):2304–47.
Eslam Ai, Nasseri S, Yadollahi B, Mesdaghinia A, Vaez F.i ,Nabizadeh R and Nazmara S , J Chem Technol Biotechnol, 83, 1447 (2008).
Kansal SK, Singh M, Sud D, Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J Hazard Mater. 2007;581. https://doi.org/10.1016/j.jhazmat.2006.07.035.
Lizama C, Freer J, Baeza J, Mansilla HD. Optimized Photodegradation of Reactive Blue 19 on TiO2 and ZnO Suspensions. Catal Today. 2002;76:2335.
Lathasree S, Rao AN, SivaSankar B, Sadasivam V and Rengaraj K, J. Mol. Catal. A: Chem, 223, 101 (2004).
Curri M, Comparelli R, Cozzoli P, Mascolo G, Agostiano A. Mater. Sci. Eng: C. 23. 2003;285.
Moshfegh AZ. Nanoparticle catalysts. J Phys D Appl Phys. 2009;42:233001. https://doi.org/10.1088/0022-3727/42/23/233001.
Zhang H, Chen G, Bahnemann DW. Photoelectrocatalytic materials for environmental applications. J Mater Chem. 2009;5089.
Mekasuwandumrong O, Pawinrat P, Praserthdam P, Panpranot J. Chem Eng. J, 164. 2010;77.
Khorram Abadi Sh, Bolshaya I, Godini H, Al-Nabi Amelishi SF, Hatami S, Goudarzi A, et al. Evaluation of the Efficiency of Advanced UV / H2O2 Oxidation Process in Removal of Ceftriaxone Antibiotic from Aquatic Environment. Lorestan Univ Med Scie. 164. 2014;1 (In persian):59.
Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for blactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–33.
Pouretedal HR, Sadegh N. Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J Water Proc Eng. 2014;1:64–73. https://doi.org/10.1016/j.jwpe.2014.03.006.
Chavoshan S, Khodadadi M, Nasseh N, Hossein Panahi A, Hosseinnejad A. Investigating the efficiency of single-walled and multi-walled carbon nanotubes in removal of penicillin G from aqueous Solutions. Environ Health Eng Manag J. 2018;5(4):187–96.
Zhang L, Song X, Liu X, Yang L, Pan F, Lv J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem Eng J 178:26–33.
Nosrati R, Olad A, Maramifar R. Degradation of ampicillin antibiotic in aqueous solution by ZnO/polyaniline nanocomposite as photocatalyst under sunlight irradiation. Environ Sci Pollut Res. 2012;19:2291–9.
Chen X, Zhou Z, Lv W, Huang T. Preparation of coreshell structured T-ZnOw/polyaniline composites via graft polymerization. Mater Chem Phys. 2009;115:258–62.
Rezaei A, Masoum B, Khataei A, Hashemian S. Effect of UV radiation intensity on photocatalytic removal of E. coli using immobilized ZnO nanoparticles. Kowsar Medi J. 2009;14(3):149–56 [Farsi].
Aksu Z, Tunç Ö. Application of biosorption for penicillin G removal: comparison with activated carbon. Process Biochem. 2005;40:831–47.
Balarak D, Mostafapour FK, Joghataei A. Experimental and kinetic studies on penicillin G adsorption by Lemna minor. Br J Pharm Res. 2016;9(5):1–10.
Noori Sepehr M, Mohebi S, Abdlollahi Vahed S. Removal of tetracycline from synthetic solution by natural LECA. J Environ Health Eng. 1:301–11.
Loncto J, Walker M, L F Nanotechnology in the water industry Nanotech L & Bus 2007;4:157.
Samadi MT, Shokoohi R, Araghchian M, Tarlani Azar M. Amoxicillin Removal from Aquatic Solutions Using Multi-Walled Carbon Nanotubes. J Mazandaran Univ Med Sci 24(117):103–15.
Li J, Deng X, Guo R, Li B, Cheng Q, Cheng X. Visible light driven photocatalytic decomposition of penicillin G by Ti3+ self-doped TiO2 nano-catalyst through response surface methodology. J Taiwan Inst Chem Eng. 2018;87:174–81. https://doi.org/10.1016/j.jtice.2018.03.033.
Dehghani M, Nasseri S, Ahmadi M, Samaei MR, Anushiravani A. Removal of penicillin G from aqueous phase by Fe+3-TiO2/UV-A process. J Environ Health Sci Eng. 2014.
Farzadkia M, Esrafili A, Baghapour MA, Dadban Shahamat Y, Okhovat N. Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalin Water Treat. 2014;52:4947–52.
Khodadadi M, Ehrampoush MH, Ghaneian MT, Allahresani A, Mahvi AH. Synthesis and characterizations of FeNi3@SiO2@TiO2 nanocomposite and its application in photo-catalytic degradation of tetracycline in simulated wastewater. J Mol Liq. 2018;255:224–32.
Acknowledgments
The present study was funded by the Deputy of Research and Technology of Birjand University of Medical Sciences with code of research 455337 and under code of ethics ir.bums.REC.1396.120 The authors hereby would like to express their gratitude and appreciation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm no conflicts of interest associated with this publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chavoshan, S., Khodadadi, M. & Nasseh, N. Photocatalytic degradation of penicillin G from simulated wastewater using the UV/ZnO process: isotherm and kinetic study. J Environ Health Sci Engineer 18, 107–117 (2020). https://doi.org/10.1007/s40201-020-00442-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00442-7