Abstract
Background
Ficus benghalensis L. is traditionally used to manage diabetes; also used in various herbal formulations, and is indicated as an insulin sensitizer. Hence, present work attempted in identifying the probable lead hits to promote glucose uptake via computational approach followed by experimental evaluation of hydroalcoholic extract of Ficus benghalensis L. bark in yeast cells.
Methods
The in vitro assay for glucose uptake was performed in the baker yeast whereas in-silico study involved retrieving the phytoconstituents from open sources, and predicting for probable targets of diabetes followed by drug-likeness score, probable side effects, and ADMET profile. Homology modeling was performed to construct the target protein glucose transporter-2. In addition, the binding affinity of each ligand with glucose transporter was predicted using AutoDock 4.2.
Results
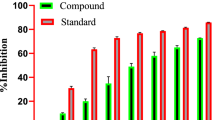
A total of 17 phytoconstituents from F. benghalensis were identified to possess the anti-diabetic effects. Among them, 4-methoxybenzoic acid scored the highest drug-likeness score and lupeol acetate had the maximum binding affinity of -8.02 kcal/mol with 9 pi-interactions via Tyr324, Phe323, Ile319, Ile200, Ile28, Phe24, and Ala451. Similarly, the extract showed the highest glucose uptake efficacy in yeast cells at 500 µg/mL.
Conclusion
Herein the present study reflected the probable activity of the phytoconstituents from F. benghalensis in promoting the glucose uptake via the in silico and in vitro approaches.





Similar content being viewed by others
Abbreviations
- 3D:
-
3 Dimensional
- ADMET:
-
Absorption, distribution, metabolism, and excretion
- ChEBI:
-
Chemical Entities of Biological Interest
- DM:
-
Diabetes Mellitus
- PDB:
-
Protein data bank
- RCSB:
-
Research Collaboratory for Structural Bioinformatics
- SMILES:
-
Simplified molecular-input line-entry system
References
LeRoith D. β-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113(6):3–11.
Ternikar SG, Patil MB, Pasha I, Dwivedi PSR. Gene ontology enrichment analysis of PPAR-γ modulators from Cassia glauca in diabetes mellitus. J Diabetes Metab Disord. 2021;20(2):1239–46.
Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254–64.
Vargas E, Podder V, Carrillo Sepulveda MA. Physiology, Glucose Transporter Type 4. [Updated 2021 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537322/
Navale AM, Paranjape AN. Glucose transporters: physiological and pathological roles. Biophys Rev. 2016;8(1):5–9.
El Bacha T, Luz M, Da Poian A. Dynamic Adaptation of Nutrient Utilization in Humans. Nat Educ. 2010;3(9):8.
Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020;472(9):1273–98.
Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2–3):121–38.
Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10(5):210–29.
Dwivedi PSR, Rasal VP. Identification of PTPN1B inhibitors from Momordica charantia and their enrichment analysis. J Phytopharmacol. 2020;9(1):38–45.
Dwivedi PS, Patil R, Khanal P, Gurav NS, Murade VD, Hase DP, Kalaskar MG, Ayyanar M, Chikhale RV, Gurav SS. Exploring the therapeutic mechanisms of Cassia glauca in diabetes mellitus through network pharmacology, molecular docking and molecular dynamics. RSC Adv. 2021;11(62):39362–75.
Duyu T, Khatib NA, Khanal P, Patil BM, Hullatti KK. Network pharmacology-based prediction and experimental validation of Mimosa pudica for Alzheimer’s disease. J Phytopharmacol. 2020;9(1):46–53.
Khanal P, Patil BM. Consolidation of network and experimental pharmacology to divulge the antidiabetic action of Ficus benghalensis L. bark. 3 Biotech. 2021;11(5):238.
Khanal P, Patil BM. In vitro and in silico anti-oxidant, cytotoxicity and biological activities of Ficus benghalensis and Duranta repens. Chin Herb Med. 2020;12(4):406–13.
Khanal P, Patil BM. Reversal of insulin resistance by Ficus benghalensis bark in fructose-induced insulin-resistant rats. J Ethnopharmacol. 2022;284:114761.
Khanal P, Patil BM. Gene set enrichment analysis of alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed. 2019;9(6):263–70.
Khanal P, Patil BM. Integration of in silico, in vitro and ex vivo pharmacology to decode the anti-diabetic action of Ficus benghalensis L. bark. J Diabetes Metab Disord. 2020;19(2):1325–37.
Dwivedi PSR, Patil VS, Khanal P, Bhandare VV, Gurav S, Harish DR, Patil BM, Roy S. System biology-based investigation of Silymarin to trace hepatoprotective effect. Comput Biol Med. 2022;142:105223.
Dwivedi PSR, Khanal P, Gaonkar VP, Rasal VP, Patil BM. Identification of PTP1B regulators from Cymbopogon citratus and its enrichment analysis for diabetes mellitus. In Silico Pharmacol. 2021;9:30.
Dwivedi PSR, Rasal VP, Kotharkar E, Khanal P. Gene set enrichment analysis of PPAR-γ regulators from Murraya odorata Blanco. J Diabetes Metab Disord. 2021;20(1):369–75.
Roy A, Dement AD, Cho KH, Kim JH. Assessing glucose uptake through the yeast hexose transporter 1 (Hxt1). PLoS One. 2015;10(3):e0121985.
Singh V, Bedi GK, Shri R. In vitro and In vivo antidiabetic evaluation of selected culinary-medicinal mushrooms (Agaricomycetes). Int J Med Mushrooms. 2017;19(1):17–25.
Rehman G, Hamayun M, Iqbal A, Ul Islam S, Arshad S, Zaman K, Ahmad A, Shehzad A, Hussain A, Lee I. In Vitro Antidiabetic Effects and Antioxidant Potential of Cassia nemophila Pods. Biomed Res Int. 2018;2018:1824790.
Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-ofconcept.’ J Ethnopharmacol. 2006;106(3):290–302.
Degtyarenko K, De Matos P, Ennis M, Hastings J, Zbinden M, McNaught A, Alcántara R, Darsow M, Guedj M, Ashburner M. ChEBI: a database and ontology for chemical entities of biological interest. Nucleic Acids Res. 2007;36(s1):D344–50.
MolSoft molecules in silico. Drug-Likeness and molecular property prediction. Available at: https://molsoft.com/mprop/
Ivanov SM, Lagunin AA, Rudik AV, Filimonov DA, Poroikov VV. ADVERPred–Web service for prediction of adverse effects of drugs. J Chem Inf Model. 2018;58(1):8–11.
Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Li W, Liu G, Tang Y. admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–9.
Cirillo VP. Mechanism of glucose transport across the yeast cell membrane. J Bacteriol. 1962;84(3):485–91.
Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996;17(5-6):490–519.
Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26(2):283–91.
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93(S1):S52–9.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(S1):S62–7.
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21(17):6275.
Vallon V, Sharma K. Sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Curr Opin Nephrol Hypertens. 2010;19(5):425–31.
Sharma H, Chandola HM. Prameha in Ayurveda: correlation with obesity, metabolic syndrome, and diabetes mellitus. Part 1-etiology, classification, and pathogenesis. J Altern Complement Med. 2011;17(6):491–6.
Sørensen SS, Christensen F, Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. X. Effect of glucose transport stimuli on the efflux of isotopically labelled calcium and 3-O-methylglucose from soleus muscles and epididymal fat pads of the rat. Biochim Biophys Acta. 1980;602(2):433–45.
Calado J, Santer R, Rueff J. Effect of kidney disease on glucose handling (including genetic defects). Kidney Int Suppl. 2011;120:S7-13.
Santer R, Schneppenheim R, Suter D, Schaub J, Steinmann B. Fanconi-Bickel syndrome–the original patient and his natural history, historical steps leading to the primary defect, and a review of the literature. Eur J Pediatr. 1998;157(10):783–97.
Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58(2):221–32.
Berger C, Zdzieblo D. Glucose transporters in pancreatic islets. Pflugers Arch. 2020;472(9):1249–72.
Reddy KP, Singh A, et al. Synthesis of novel triterpenoid (lupeol) derivatives and their in vivo antihyperglycemic and antidyslipidemic activity. Bioorganic Med Chem Lett. 2009;19(15):4463–6.
Kottireddy S, Koora S. Lupeol exerts its antidiabetic activity through insulin receptor and glucose transporter-4 in gracilis muscle in high fat and sucrose-induced diabetic rats. Drug Invent Today. 2019;11(9):2258–64.
Shreenithi S, Vishnupriya V, Ponnulakshmi R, Gayathri R, Madhan K, Shyamaladevi B, Selvaraj J. In silico and in vivo approach to identify the antidiabetic activity of lupeol. Drug Invent Today. 2019;11(5):1113–6.
Oyinloye BE, Adekiya TA, Aruleba RT, Ojo OA, Ajiboye BO. Structure-Based Docking Studies of GLUT4 towards Exploring Selected Phytochemicals from Solanum xanthocarpum as a Therapeutic Target for the Treatment of Cancer. Curr Drug Discov Technol. 2019;16(4):406–16.
Cersosimo E, Triplitt C, Solis-Herrera C, et al. Pathogenesis of Type 2 Diabetes Mellitus. [Updated 2018 Feb 27]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279115/
Acknowledgements
The authors are thankful to Principal KLE College of Pharmacy Belagavi, KLE Academy of Higher Education and Research (KAHER) Belagavi.
Funding
This work has not received any funds from any national or international agencies in any financial or non-financial means to declare.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors of this manuscript declare that they do not have any conflict of interest in any financial means. All the authors have read and approved this manuscript.
Ethical statement
This work does not include any animal or human participation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madiwalar, V.S., Dwivedi, P.S.R., Patil, A. et al. Ficus benghalensis promotes the glucose uptake- Evidence with in silico and in vitro. J Diabetes Metab Disord 21, 429–438 (2022). https://doi.org/10.1007/s40200-022-00989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-00989-2