Abstract
Introduction
Type 1 Diabetes Mellitus (T1DM) is an auto immune reaction against insulin secreting beta cells. Exogenous insulin administration is the only standard treatment for T1DM. However, despite tight glycemic control many patients will develop chronic life-threatening complications. Recently, stem cell transplantation has been suggested as a novel treatment for eliminating the beta cell damage and promoting their regeneration by modulating auto-immunity. To our knowledge; this is the first preliminary report of placenta derived MSCs (PLMSCs) transplantation in juvenile T1DM.
Method
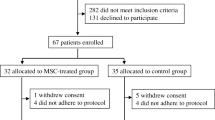
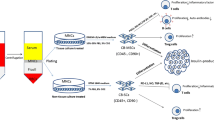
An Open label non-randomized phase 1 clinical trial was designed to evaluate the safety of PLMSCs transplantation in new onset juvenile T1DM (IRCT20171021036903N2). PLMSCs were manufactured in our clean room facility using a Xeno-free/GMP compliant protocol. The first series of patients (n = 4) received one dose of1 × 106 PLMSCs/kg intravenously. Diabetic clinical and laboratory parameters and side effects were evaluated weekly for the first month, monthly for 6 months, and then every 3 month till 1 year.
Results
Serious adverse events were not seen during 1 year follow-up. Partial remission and hypoglycemic attacks were happened one month after transplantation in two patients. ZnT8-Ab decreased till month 3 and then increased again in all patients. Anti Gad-Ab decreased till month 3 of follow up then increased.
Discussion
This preliminary report of our phase I clinical trial demonstrated the short term safety of PLMSCs transplantation in juvenile T1DM. To prove the long term safety and probable efficacy of this treatment more investigations are needed.
Trial registration
Iranian Registry of Clinical Trials: IRCT20171021036903N2



Similar content being viewed by others
References
Control D, Group CTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med . 1993;329(14):977–86.
Skrivarhaug T, et al. Low risk of overt nephropathy after 24 year of childhood-onset type 1 diabetes mellitus (T1DM) in Norway. Pediatr Diabetes. 2006;7(5):239–246.
Nordwall M, et al. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of Type 1 diabetes—the Linköping Diabetes Complications Study. Diabetologia. 2004;47(7):1266–72.
Meunier L, et al. Age, male gender, and social deprivation are associated with a lower rate of insulin pump therapy initiation in adults with type 1 diabetes: a population-based study. Diabetes Technol Ther. 2021;23(1):8–19.
Couper JJ, et al. ISPAD clinical practice consensus guidelines 2018: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2018;19:20–7.
Zhang J, et al. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver. 2018;12(1):73.
Wang D, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–77.
Cui Y, et al. Metabolomic analysis of the effects of adipose-derived mesenchymal stem cell treatment on rats with sepsis-induced acute lung injury. Front Pharmacol. 2020;11:902.
Cho J, et al. A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 2018;7(4):82.
Xv J, et al. Mesenchymal stem cells moderate immune response of type 1 diabetes. Cell Tissue Res. 2017;368(2):239–48.
Khatri R, et al. Mesenchymal stem cells promote pancreatic β-cell regeneration through downregulation of FoxO1 pathway. Stem Cell Res Ther. 2020;11(1):1–14.
Madani S, et al. Safety and efficacy of hematopoietic and mesanchymal stem cell therapy for treatment of T1DM: a systematic review and meta-analysis protocol. Syst Control Found Appl. 2018;7(1):23–3.
Lalu MM, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559-9.
El-Badawy A, El-Badri N. Clinical efficacy of stem cell therapy for diabetes mellitus: a meta-analysis. PLoS One. 2016;11(4):e0151938.
Murray JD, Doty A, Jones N. A new ball for an old trick: paramagnetic cell sorting of human mesenchymal stem cells from adipose tissue. J Stem Cell Rep. 2019;1:1–11.
Kim MJ, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346(1):53–64.
Queckbörner S, et al. Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res Ther. 2020;11(1):1–15.
Liu W, et al. Human placenta-derived adherent cells induce tolerogenic immune responses. Clin Transl Immunol. 2014;3(5):e14.
Zhang L, et al. Application potential of mesenchymal stem cells derived from Wharton’s jelly in liver tissue engineering. Biomed Mater Eng. 2015;25(1 Suppl):137–43.
Wang L, et al. Adipose tissue-derived stem cells from Type 2 diabetics reveal conservative alterations in multidimensional characteristics. Int J Stem Cells. 2020;13(2):268–78.
Araújo AB, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017;19(5):577–85.
Francki A, et al. Angiogenic properties of human placenta-derived adherent cells and efficacy in hindlimb ischemia. J Vasc Surg. 2016;64(3):746-56.e1.
Han ZC, et al. New insights into the heterogeneity and functional diversity of human mesenchymal stem cells. Biomed Mater Eng. 2017;28(s1):S29-s45.
Aronsson-Kurttila W, et al. Placenta-derived decidua stromal cells for hemorrhagic cystitis after stem cell transplantation. Acta Haematol. 2018;139(2):106–14.
Sadeghi B, et al. Safety and efficacy of placenta derived decidua stromal cells in experimental studies and clinical settings. Cytotherapy. 2017;19(5):S193.
Baughman RP, et al. Placenta-derived mesenchymal-like cells (PDA-001) as therapy for chronic pulmonary sarcoidosis: a phase 1 study. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(2):106–14.
Lublin FD, et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: a randomized, placebo-controlled, multiple-dose study. Mult Scler Relat Disord. 2014;3(6):696–704.
Chambers DC, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19(7):1013–8.
Aghayan HR, et al. GMP-compliant production of human placenta-derived mesenchymal stem cells. Stem Cells and Good Manufacturing Practices. New York: Humana; 2020. p. 213–225.
Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Chiang JL, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54.
Dave S, et al. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin Exp Med. 2013;15(1):41–5.
Zhang X, et al. Acute response of peripheral blood cell to autologous hematopoietic stem cell transplantation in type 1 diabetic patient. PLoS One. 2012;7(2):e31887.
Pruessner JC, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31.
Esfahani EN, et al. Administration of autologous mesenchymal stem cell transplantation for treatment of Type 1 diabetes mellitus. Iran J Public Health. 2015;44:55–68.
Esfahani EN, et al. The effect of fetal liver-derived cell suspension allotransplantation on patients with wolfram syndrome: the first year of follow-up. Iran J Public Health. 2015;44:69–76.
Ghodsi M, et al. Insulin Independence after fetal liver-derived cell suspension allotransplantation in patients with Type 1 diabetes: a pilot study. Iran J Public Health. 2015;44:27–35.
Ghodsi M, et al. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran . 2012;50(8):541–6.
Tootee A, et al. Application of allotransplantation of fetal liver-derived stem-cells for treatment of Type 1 Diabetes: a single-arm, phase 3 clinical trial. Iran J Public Health. 2015;44:36–41.
Ringden O, et al. Placenta-derived decidua stromal cells for treatment of severe acute graft-versus-host disease. Stem Cells Transl Med. 2018;7(4):325–31.
Max Andersen ML, et al. Partial remission definition: validation based on the insulin dose-adjusted HbA1c (IDAA1C) in 129 Danish children with new-onset type 1 diabetes. Pediatr Diabetes. 2014;15(7):469–76.
Haller MJ, Long SA, et al. Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA(1c), and Increases regulatory to conventional T-cell ratios in new-onset Type 1 diabetes two-year clinical trial data. Diabetes. 2019;68(6):1267–76.
Besser REJ, et al. Lessons From the Mixed-Meal Tolerance Test. Use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care. 2013;36(2):195–201.
Xu P, et al. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35(10):1975–80.
Vipin VP, et al. High prevalence of idiopathic (islet antibody-negative) type 1 diabetes among Indian children and adolescents. Pediatr Diabetes. 2020;22(1):47–51.
Mikk ML, et al. HLA-DR‐DQ haplotypes and specificity of the initial autoantibody in islet specific autoimmunity. Pediatric Diabetes. 2020;21(7):1218–26.
IDF I. Global IDF/ISPAD guideline for diabetes in childhood and adolescence. IDF available at: https://www.ispad.org/sites/default/files/resources/files/idf-ispad_diabetes_in_childhood_and_adolescence_guidelines_2011_0.pdf (2011). Accessed 10/04/2017.
Pers Y-M, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: A phase i dose‐escalation trial. Stem Cells Transl Med . 2016;5(7):847–56.
Kim H, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010;468(3):190–4.
Ringden O, Le Blanc K. Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract Res Clin Haematol. 2011;24(1):65–72.
Acknowledgements
The authors should thank Mrs. Fatemeh vakili for her assistance in placenta procurement, Mrs. Zahra Azizkhani, the head nurse of pediatric endocrinology ward, and other nurses of the endocrinology ward of children medical center of Tehran who survived the patients.
Funding
This study funding has been provided by Endocrine and Metabolism research Institute of Tehran University of Medical Science. The sponsor of the research has no role in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The funder will pay no funding for paper publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists for none of the authors of this study.
Ethical approval
Ethical approval for this study was obtained from *Tehran University Ethics Committee Review Board (APPROVAL NUMBER/ID: IR.TUMS.VCR.REC.1396-4367)*.
Informed consent
Written informed consent was obtained from all placenta donor subjects before the study, from all diabetic subjects before the study, and also from all diabetic children's legally authorized representatives before the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Template of final product’sCertificate of Analysis

Rights and permissions
About this article
Cite this article
Madani, S., Setudeh, A., Aghayan, H.R. et al. Placenta derived Mesenchymal Stem Cells transplantation in Type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metab Disord 20, 1179–1189 (2021). https://doi.org/10.1007/s40200-021-00837-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00837-9