Abstract
Background
In front of the polymorphic bacterial ecology and antibiotic resistance in diabetic patients with foot infections and good patient care, collaboration between clinicians and microbiologists is needed to improve assessment and management of patients with this pathology.
Objective
This study was designed to characterize the bacterial ecology of diabetic foot infection (DFIs) and to determine the different mechanisms of resistance involved.
Methods
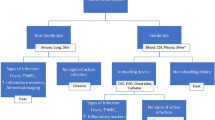
In this study bacterial strains and antibiotic resistance profiles were determined from diabetic foot infections patients (n = 117). The identification of resistance mechanisms, such as penicillinase and/or extended-spectrum β-lactamase production (ESBL), methicillin-resistant Staphylococcus aureus (MRSA) and efflux pump over-expression were performed.
Results
A high prevalence of Gram-negative bacteria (61%) with Escherichia coli, and other Enterobacteriaceae and Pseudomonas aeruginosa being the predominant isolates. Gram positive bacteria mainly represented by Staphylococcus aureus accounted for 39% of the isolates. 93.5% of the Enterobacteriaceae were resistant to, at least, one molecule in the β-lactam family, while the majority of the Staphylococci were resistant to penicillin G and tetracycline (93.3% and 71.7%). The majority of non-fermenting Gram negative bacteria were also resistant to fluoroquinolones. β-lactamase detection tests revealed the presence of extended-spectrum β-lactamase in 43.5% of the Enterobacteriaceae, while methicillin-resistant Staphylococcus aureus represented 18.2% of the isolates. Additionally, 50.9% of non-fermenting Gram negative bacteria were overproducing efflux pumps.
Conclusion
All Acinetobacter Baumannii were Multidrug-Resistant (MDR), as the majority of Staphylococci, and Enterobacteriaceae. These results should be taken into account by the clinician in the prescription of probabilistic antibiotic therapy in this context.




Similar content being viewed by others
References
Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Saudi J Kidney Dis Transpl. 2010;21:217–21.
Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12:357–70.
Eszter PV, Bottle A, Edmonds ME, Valabhji J, Majeed A, Millett C. Changes in the incidence of lower extremity amputations in individuals with and without diabetes in England between 2004 and 2008. Diabetes Care. 2010;33(12):2592–7.
Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J AntimicrobChemother. 2005;55:143–9.
Lipsky BA, Berendt AR, Embil J, De Lalla F. Diagnosing and treating diabetic foot infections. Diabetes Metab Res Rev. 2004;20:56–64.
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. J Am Med Assoc. 2005;293:217–28.
Williams DT, Hilton JR, Harding KG. Diagnosing foot infections in diabetes. Clin Infect Dis. 2004;39:83–6.
Zubair M, Malik A, Ahmad J. Clinico-bacteriology and risk factors for the diabetic foot infection with multidrug resistant microorganisms in North India. Biol Med. 2010;2:22–34.
Citron DM, Goldstein EJC, Merriam CV, Lipsky BA, Abramson MA. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J ClinMicrobiol. 2007;45:2819–28.
Damir A. Diabetic Foot Infections. JIMSA. 2011;24:4.
Davies J, Davies D. Origins and evolution of antibiotic resistance. MicrobiolMolBiol Rev. 2010;74:417–33.
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Lipsky BA, Aragon-Sanchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32:45–74.
Société de pathologie infectieuse de langue française SPILF. Recommandations pour la pratique clinique Prise en charge du pied diabétique infecté. Médecine et maladies infectieuses. 2007;37:26–50.
Rondas AA, Schols JM, Halfens RJ, Stobberingh EE. Swab versus biopsy for the diagnosis of chronic infected wounds. Advances in Skin Wound Care. 2013;5:211–9.
EUCAST-The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. 2016;Version.1.0. http://www.eucast.org
European Centre for Disease Prevention and Control (ECDC) & European Medicines Agency (EMEA). The bacterial challenge: time to react. 2009; Stockholm & London. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf
Tzelepi E, Giakkoupi P, Sofianou D, Loukova V, Kemeroglou A, Tsakris A. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J Clin Microbiol. 2000;38:542–6.
Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement. 2015;M100-S25, Wayne, PA.
MARRA M.A. Description par E-Test de la sensibilité des souches dakaroises de S.aureus. Thèse. 1998 ; Université de Dakar.
Mullié C, Bouharkat B, Guiheneuf R, Serra C, Tir Touil-Meddah A and Sonnet P. Efflux pumps in Acinetobacter baumannii: role in antibiotic resistance and interest of efflux pump inhibitors as additional therapeutic weapons. Microbiology Book Series. #6: “Antimicrobial research. 2017;572–583.
Sonnet P, Izard D, Mullié C. Prevalence of efflux-mediated ciprofloxacin and levofloxacin resistance in recent clinical isolates of Pseudomonas aeruginosa and its reversal by the efflux pump inhibitors 1-(1-naphthylmethyl)-piperazine and phenylalaninearginine- β-naphthylamide. Int J Antimicrob Agents. 2012;39:77–80.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. ClinMicrobiol Infect. 2012;18:268–81.
Sanjith S, Sahu M, Chaddha R, Pathrose E, Bal A, Bhalekar P, et al. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz J Microbiol. 2017;49:401–6.
Murali TS, Kavitha S, Spoorthi J, Bhat DV, Bharath Prasad AS, Upton Z, et al. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J. Med. Microbiol. 2014;63:1377–85.
Anvarinejad M, Pouladfar G, Japoni A, Bolandparvaz S, Satiary Z, Abbasi P, et al. Isolation and antibiotic susceptibility of the microorganisms isolated from diabetic foot infections in Nemazee hospital. Southern Iran J Pathog. 2015;2015:1–7. https://doi.org/10.1155/2015/328796.
Abdulrazak A, Bitar ZI, Al Shamali AA, Mobasher LA. Bacteriological study of diabetic foot infections. J Diabetes Complicat. 2005;19:138–41.
Osariemen IJ, Olowu SS, Adevbo E, Omon EE, Victoria O, Imuetinyan EJ, et al. Aerobic bacteria associated with diabetic wounds in patients attending clinic in a rural community in Nigeria. Glob Res J Microbiol. 2013;3:8–11.
Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol. 2010;130:38–48.
Lipsky BA, Berendt AR, Cornia PB, et al. Infectious diseases Society of America clinical practice guideline for the diagnostic and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:132–73.
Al-Hamead HA, Ibrahim Al-Metwally R, Attia Khaled M, Moawad Mahmoud M, El-ramah Ayman F, Shahin Mohamed M, et al. Bacteriological study of diabetic foot infection in Egypt. Journal of the Arab Society for Medical Research. 2013;8:26–32.
International Working Group on the Diabetic Foot-IWGDF (2011) International Consensus on the Diabetic Foot and Practical Guidelines on the Management and the Prevention of the Diabetic Foot. https://www.sfdiabete.org/sites/www.sfdiabete.org/files/files/Recos-Référentiels/Recommandations_IWGDF_2011.pdf
Hartemann-Heurtier A, Robert J, Jacqueminet S, Ha van G, Golmard JL, Jarlier V, et al. Diabetic foot ulcer and multidrug-resistant organisms: risk factors and impact. Diabet Med. 2004;21:710–5.
Xiaoying Xie, Yunwen Bao, Lijia Ni, Dan Liu, Shaona Niu, Haixiong Lin, et al. Bacterial profile and antibiotic resistance in patients with diabetic foot ulcer in Guangzhou, Southern China: Focus on the Differences among Different Wagner’s Grades, IDSA/IWGDF Grades, and Ulcer Types. International Journal of Endocrinology. 2017; https://doi.org/10.1155/2017/8694903
Zubair M, Malik A, Ahmad J. Clinicomicrobiological study and antimicrobial drug resistance profile of diabetic foot infections in North India. Foot (Edinb). 2011;21(1):6–14.
Banashankari GS, Rudresh HK, Harsha AH. Prevalence of gram negative Bacteria in diabetic foot-a Clinico-microbiological study. Al Ameen J Med Sci. 2012;5(3):224–32.
Shakil S, Khan AU. Infected foot ulcers in male and female diabetic patients: a clinico-bioinformative study. Ann ClinMicrobiolAntimicrob. 2010;9:2.
Perim MC, Borges Jda C, Celeste SR, Orsolin Ede F, Mendes RR, et al. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Bras Med Trop. 2015;48(5):546–54.
El-Sheikh NA, Badawi MM, Mahran MH, Abdelfattah MM. Bacteriology of diabetic foot ulcer among an Egyptian population: a retrospective study. World Journal of Medical Sciences. 2014;10(4):494–502.
Razavi Nikoo H, Ardebili A, Mardaneh J. Systematic review of antimicrobial resistance of clinical Acinetobacter baumannii isolates in Iran: an update. Microb Drug Resist. 2017;23(6):744–56.
Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55.
Soltani B, Heidari H, Ebrahim-Saraie HS, Hadi N, Mardaneh J, Motamedifar M. Molecular characteristics of multiple and extensive drug-resistant Acinetobacter baumannii isolates obtained from hospitalized patients in southwestern Iran. Infez Med. 2018 Mar 1;26(1):67–76.
Jahansepas A, Aghazadeh M, Rezaee MA, Hasani A, Sharifi Y, Aghazadeh T, et al. Occurrence of Enterococcus faecalis and Enterococcus faecium in various clinical infections: detection of their drug resistance and virulence determinants. Microb Drug Resist. 2018;24(1):76–82.
Nasir CW, Rabia B, Muhsin J, Jason J, Jamal Z, Saadia A. Clinico-microbiological study and antibiotic resistance profile of mecA and ESBL gene prevalence in patients with diabetic foot infections. ExpTher Med. 2016;11(3):1031–8.
Joseph WS, Lipsky BA. Medical therapy of diabetic foot infections. J Am Podiatr Med Assoc. 2010;100:395–400.
Sekhar SM, Vyas N, Unnikrishnan MK, Rodrigues GS, Mukhopadhyay C. Antimicrobial susceptibility pattern in diabetic foot ulcer: a pilot study. Ann Med Health Sci Res. 2014;4(5):742–5.
Shankar EM, Mohan V, Premalatha G, Srinivasan RS, Usha AR. Bacterial etiology of diabetic foot infections in South India. Eur J Intern Med. 2005;16(8):567–70.
Djahmi N, Messad N, Nedjai S, et al. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University hospital. ClinMicrobiol Infect. 2013;19:398–404.
Samir V, Raymond A. Que signifie «bêtalactamases à spectre élargi» en pratique? Rev Med Suisse. 2009;5:1991–4.
Al Benwan K, Al Mulla A, Rotimi VO. A study of the microbiology of diabetic foot infections in a teaching hospital in Kuwait. J Infect Public Health. 2012;5(1):1–8.
Dang CN, Prasad YD, Boulton AJ, Jude FB. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20:159–61.
Tentolouris N, Petrikkos G, Vallianou N, Zachos C, Daikos GL, Tsapogas P, et al. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. ClinMicrobiol Infect. 2006;12:186–9.
Kumar VA, Steffy K, Chatterjee M, Sugumar M, Dinesh KR, Manoharan A, et al. Detection of oxacillin-susceptible mecA-positive Staphylococcus aureus isolates by use of chromogenic medium MRSA ID. J ClinMicrobiol. 2013;51:318–9.
Hiroshi N. Multidrug Resistance in Bacteria. Annu Rev Biochem 2009;78: 119–146.
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious disease society of America for the treatment of methicillin- resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:18–55.
Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55:947–53.
Pan YP, Xu YH, Wang ZX, Fang YP, Shen JL. Overexpression of MexAB-OprM efflux pump in carbapenem-resistant Pseudomonas aeruginosa. Arch Microbiol. 2016;198(6):565–71.
Aires Julio. Les systèmes d’efflux actifs bactériens : Caractérisation et modélisation pour quelles Perspectives. Bull. Acad. Vét. France– 2011 - Tome 164 - N°3 http://documents.irevues.inist.fr/bitstream/handle/2042/48096/AVF_2011_3_265.pdf?sequence=1
Wang S-H, Sun Z-L, Guo Y-J, Yang B-Q, Yuan Y, Wei Q, et al. Methicillin-resistant Staphylococcus aureus isolated from foot ulcers in diabetic patients in a Chinese care hospital: risk factors for infection and prevalence. J Med Microbiol. 2010;59:1219–24.
Jain SK, Barman R. Bacteriological profile of diabetic foot ulcer with special reference to drug-resistant strains in a tertiary Care Center in North-East India. Indian Journal of Endocrinology and Metabolism. 2017;21(5):688–94.
Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC, Chaudhry RA. Clinicomicrobiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29:1727–32.
Gaëlle C, Françoise D, Philippe T, Igor T, Richard B, et al. Émergence des infections monomicrobiennes à Staphylocoque doré méthicilline-résistant dans les ostéites du pied diabétique. Presse Med. 2007;36:851–8.
Fırat O, Kaan G, Çelikİlhami BDZ. Evaluation of major and minor lower extremity amputation in diabetic foot patients, Erdal UZUN. Turk J Med Sci. 2017;47:1109–16.
Dan L, Karim G, Benjamin K, Elodie VD, Benedikt H, et al. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int. J. Infect. Dis. 2017;59:61–4.
Goli HR, Nahaei MR, Rezaee MA, Hasani A, Samadi Kafil H, Aghazadeh M, et al. Contribution of mexAB-oprM and mexXY (−oprA) efflux operons in antibiotic resistance of clinical Pseudomonas aeruginosa isolates in Tabriz. Iran Infect Genet E. 2016;45:75–82.
Nakashima R, Sakurai K, Yamasaki S, Hayashi K, Nagata C, Hoshino K, et al. Structural basis for the inhibition of bacterial multidrug exporters. Nature. 2013;500:102–6.
Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria- an update. Drugs. 2009;69:1555–623.
Abdi-Ali A, Rahmani-Badi A, Falsafi T, Nikname V. Study of antibiotic resistance by efflux in clinical isolates of Pseudomonas aeruginosa. Pak J Biol Sci. 2007;10:924–7.
Kuo HY, Chang KC, Kuo JW, Yueh HW, Liou ML. Imipenem: a potent inducer of multidrug resistance in Acinetobacter baumannii. Int J Antimicrob Agents. 2012;39:33–8.
Pannek S, Higgins PG, Steinke P, Jonas D, Akova M, Bohnert JA, et al. Multidrug efflux inhibition in Acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J Antimicrob Chemother. 2006;57:970–4.
Bruno S. Lopes, Lucía Gallego, Sebastian G. B. Amyes. Multi-drug resistance profiles and the genetic features of Acinetobacter baumannii isolates from Bolivia. J Infect Dev Ctries. 2013;7(4):323–8.
Lin L, Ling B-D, Li X-Z. Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii–Acinetobacter calcoaceticus complex. Int J Antimicrob Agents. 2009;33:27–32.
Ardebili A, Talebi M, Azimi L, Rastegar LA. Effect of efflux pump inhibitor carbonyl cyanide 3-Chlorophenylhydrazone on the minimum inhibitory concentration of ciprofloxacin in Acinetobacter baumannii clinical isolates. Jundishapur Journal of Microbiology. 2014;7:e8691. https://doi.org/10.5812/jjm.8691.
Wieczorek P, Sacha P, Hauschild T, Zórawski M, Krawczyk M, Tryniszewska E. Multidrug resistant Acinetobacter baumannii--the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia HistochemCytobiol. 2008;46(3):257–67.
Hosseinzadeh Z. Emerge of blaNDM-1 and blaOXA-48-like harboring carbapenem-resistant Klebsiella pneumoniae isolates from hospitalized patients in southwestern Iran.J Chin Med Assoc. 2018 Jun;81(6):536–540.
Anvarinejad M, et al. Diabetic foot infections: antibiotic susceptibility patterns and determination of antibiotic cross-resistance in clinical isolates of Enterococcus species during 2012-2014 in shiraz, Iran. Arch Pediatr Infect Dis. 2017;5(2):e37680.
Acknowledgments
Part of this work was funded by the Ministry of Higher Education and Scientific Research under the Hubert Curien Tassili Partnership between Algeria and France (project 16MDU974/35118Z).
Author information
Authors and Affiliations
Contributions
BB: technical handling of the samples and article writing; CM: design and supervision of efflux pump inhibition experiments and article writing, NC:, BM:, ATT: design and supervision of the clinical study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent
Informed consent was obtained from all individual patients included in the study article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouharkat, B., Tir Touil, A., Mullié, C. et al. Bacterial ecology and antibiotic resistance mechanisms of isolated resistant strains from diabetic foot infections in the north west of Algeria. J Diabetes Metab Disord 19, 1261–1271 (2020). https://doi.org/10.1007/s40200-020-00639-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00639-5