Abstract
Purpose of Review
In this review, we discuss the key molecular and clinical developments in VHL disease that have the potential to impact on the natural history of the disease and improve patient outcomes.
Recent Findings
Identifiable mutations in VHL underlie most cases of VHL and define clear genotype-phenotype correlations. Detailed clinical and molecular characterisation has allowed the implementation of lifelong screening programmes that have improved clinical outcomes. Functional characterisation of the VHL protein complex has revealed its role in oxygen sensing and the mechanisms of tumourigenesis that are now being exploited to develop novel therapies for VHL and renal cancer.
Summary
The molecular and cellular landscape of VHL-associated tumours is revealing new opportunities to modify the natural history of the disease and develop therapies. Drugs are now entering clinical trials and combined with improved clinical and molecular diagnosis, and lifelong surveillance programmes, further progress towards reducing the morbidity and mortality associated with VHL disease is anticipated.
Similar content being viewed by others
Introduction
von Hippel-Lindau (VHL) disease is an autosomal dominant multisystem cancer predisposition disorder caused by germline mutations in the VHL tumour suppressor gene [1, 2]. Up to 20% of cases are due to de novo pathogenic variants and therefore have no family history [3]. VHL disease demonstrates age-dependent and incomplete penetrance and variable expression [4,5,6]. The most common tumours associated with VHL disease are retinal and central nervous system haemangioblastomas (in the cerebellum, brain stem or spinal cord), clear cell renal cell carcinoma (cRCC), phaeochromoctyma/paraganglioma (PPGL), non-secretory pancreatic neuroendocrine tumours and endolymphatic sac tumours [4, 6] (see Table 1). Visceral (renal, pancreatic and epididymal) cysts occur frequently and may suggest the diagnosis when they are detected in combination with a VHL-related tumour [6].
Comprehensive reviews of the historical, clinical and molecular aspects of VHL disease and the VHL tumour suppressor gene (TSG) have been published in the past 5 years [1, 2]. In this brief review, we discuss some of the key molecular and clinical aspects of VHL disease and highlight some of the recent relevant literature focussing on developments that have the potential to impact on the natural history of the disease and improve patient outcomes.
Genetics of VHL Disease
VHL disease is caused by monoallelic pathogenic variants in the VHL tumour suppressor gene which maps to chromosome 3p25 [1, 7]. VHL disease causing VHL variants are heterogeneous and include single or multi-exon deletions (30–40% of cases), truncating mutations (~ 30% of case) and missense variants (~ 30%) [5, 8]. The VHL gene encodes two proteins, a full length 213 amino acid protein and a shorter one that is translated from a second initiation site at codon 54 (pVHL30 and pVHL19 respectively). Loss of function variants that impact on both proteins are pathogenic and no phenotype has been associated with variants that only alters the longer form [8] (Fig. 1). Genotype-phenotype correlations are a well- recognised feature of VHL disease (see Table 2). VHL type 1 disease is associated with the development of retinal and CNS haemangioblastomas and cRCC but rarely PPGL and pathogenic variants in this group of patients are usually exon deletions, truncating variants or missense variants that are predicted to destabilise pVHL [1, 2]. VHL type 2 disease is associated with frequent PPGL and the pathogenic variants detected are most often missense substitutions at pVHL surface residues. Type 2 VHL disease is further subdivided into type 2A (PPGL, haemangioblastomas but not RCC), type 2B (PPGL, haemangioblastomas and RCC) and type 2C (PPGL only) and specific missense substitutions can be found in each subtype [1, 2]. A further genotype-phenotype correlation is the association of congenital polycythaemia with specific biallelic missense variants (e.g. p.Arg200Trp) [9].
Distribution of germline variants across the VHL coding sequence in patients diagnosed with von Hippel-Lindau disease. Data extracted from the missense and in frame deletion (“missense/IFD”) and nonsense/frameshift variants (“truncating”). Data extracted from http://vhldb.bio.unipd.it/mutations [8]
Clinical diagnostic criteria for VHL disease (e.g. a typical VHL type tumour in an individual with a family history of VHL disease or in sporadic cases two haemangioblastomas or a haemangioblastoma and a visceral tumour) can lead to under or late diagnosis of VHL disease particularly in patients without a family history but the availability of routine molecular genetic testing for more than two decades has therefore enabled accurate and earlier diagnosis [6]. A molecular diagnosis can be made 95% or more of individuals with a clinical diagnosis of VHL disease. To date, there is no evidence for locus heterogeneity and, with the increasing use of next generation sequencing techniques, the suspicion that a proportion of cases without a molecular diagnosis are mosaic for a pathogenic variant in peripheral blood has been confirmed. Interestingly, though it might be predicted that mosaic cases might more mildly affected, low levels of mosaicism have been reported in individuals with a classical VHL disease phenotype [10, 11]. Less commonly, promoter region variants have been reported to be pathogenic [12]. Recently, intronic variants or synonymous variants in exon 2 (of three VHL exons) have been reported to result in dysregulated splicing and cause VHL disease, familial phaeochromocytoma and, when homozygous/compound heterozygous state, with erythrocytosis [13•, 14]. These novel findings suggest that mutation negative individuals in whom VHL disease is strongly suspected should be now re-evaluated for further genetic testing. Somatic mutation testing may eventually help define disease in the very small number of cases with no evidence of mosaicism in blood.
Mechanisms of Tumourigenesis in VHL Disease and Sporadic VHL-Related Tumours
Prior to the identification of the VHL TSG, statistical analysis had demonstrated that the ages at onset of cerebellar haemangioblastomas and RCC in VHL disease and sporadic cases were consistent with a one-hit and two-hit model of tumourigenesis (as in retinoblastoma) [15]. Subsequent molecular studies confirmed this with somatic inactivation occurring in most clear cell RCC and in tumours from patients with VHL disease, the somatic inactivation of VHL is present as second hit [16,17,18,19]. CNS haemangioblastomas comprise a mixture of stromal cells, pericytes, endothelial cells and lymphocytes. Though the neoplastic component is the stromal cells, these only account for ~ 20% of the tumour [20]. Deep sequencing has demonstrated that, as in sporadic cRCC, somatic VHL inactivation can be detected within the stromal cells in most sporadic haemangioblastomas [21].
Phenotypic variability is a prominent feature of VHL disease and, in addition to allelic heterogeneity, genetic modifier and stochastic events have been implicated [5, 22]. Analysis of somatic variants from multiple cRCC from individuals with VHL disease has confirmed that they are clonally independent but also that within-patient patterns are identifiable, implicating a role for genetic background and environmental effects in influencing the acquisition of somatic mutations [23]. Whilst biallelic VHL inactivation is apparently necessary for tumourigenesis, in most sporadic clear cell RCC (cRCC, the most common type of RCC), current models of tumourigenesis specify that VHL inactivation alone is not sufficient to for tumourigenesis. In addition to somatic VHL mutations, most cRCC demonstrates loss of the short arm of chromosome 3 that extends far centromeric (e.g. to 3p12 or 3p14) to VHL at 3p25 [24,25,26,27]. These large 3p deletions will result in loss of one allele of other 3p TSGs such as the epigenetic regulators BAP1, PBRM1 and SETD2 and somatic mutations in these genes can be detected in many sporadic cRCC [28,29,30,31]. A number of recent studies have looked at the relationship between VHL and BAP1 or PBRM1 inactivation. In a mouse model, a combination of Vhl−/− and Bap1+− deficiency in nephron progenitor cells resulted in multiple renal cysts and tumours (similar to kidneys in VHL disease) whereas Vhl−/− mice did not develop cRCC [32]. Similarly, in cellular and mouse models, loss of PBRM1/Pbrm1 in addition to VHL/Vhl was oncogenic [33,34,35].
In a seminal study, Mitchell et al. [36••] reported that the most frequent cause of chromosome 3p loss in cRCC is a chromothrypsis-associated rearrangement between 3p and 5q that resulted in loss of 3p and gain of 5q. It was estimated that the t(3;5) initiating event occurred early in life (childhood/adolescence) in the majority of cases, preceding other driver mutations and 30–50 years before the diagnosis of RCC [36••]. In a reanalysis of genome data from VHL disease RCC, there was a similar frequency of t(3;5) and age-related rate of accumulation of somatic mutations as in sporadic cRCC [23, 36••]. Though it was predicted that both VHL-related RCC and sporadic cRCC would be initiated by biallelic VHL inactivation [15], the first event in VHL RCC is a germline VHL mutation whilst in sporadic cRCC, the first event appears to be 3p loss and then a somatic VHL mutation [36••]. Intriguingly, it has been suggested that because of the long latency between 3p loss and clinical cRCC, it might be possible to prevent the later development of cRCC by therapeutic targeting of cells harbouring a 3p deletion [36••].
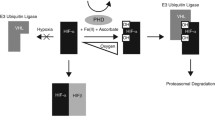
pVHL Function and Oncogenic Drivers of Tumourigenesis
Following the identification of the VHL TSG in 1993, there were no clues to likely pVHL function from the primary protein sequence but, after reports that pVHL-deficient cRCC cells showed abnormal levels of hypoxia-responsive mRNAs (e.g. VEGF, PDGFB, GLUT1) in both normoxic and hypoxic conditions, Maxwell et al. [37] demonstrated that pVHL had a critical role in regulating the expression of the α-subunits of the hypoxia-inducible transcription factors, HIF-1 and HIF-2 [1, 38]. Further studies have shown that pVHL functions as the target binding component of an E3 ubiquitin ligase complex that also contains elongin C, elongin B, cullin 2 and RBX1 (VCB-CR complex) [39,40,41,42,43] (see Fig. 2). In normoxic conditions, the β-domain of pVHL binds to the oxygen-dependent degradation domains of HIF-1α and HIF-2α resulting in polyubiquitination and proteasomal degradation of the HIF α-subunits [37,38,39,40,41,42,43]. The ability of pVHL to bind to the HIF α-subunits is dependent on hydroxylation of two conserved proline residues. Oxygen is an essential cofactor for the proline hydroxylation enzymes (PHD1, PHD2, PHD3) and under hypoxic conditions, the HIF-α subunit proline residues are not hydroxylated, pVHL is therefore unable to bind and HIF-1 and HIF-2 are stably expressed and hypoxic gene response pathways (> 200 genes) are activated [44, 45]. Biallelic VHL inactivation in tumour cells mimics the effect of hypoxia and the resulting transcriptional response is thought to drive both angiogenesis and oncogenesis. HIF-1 and HIF-2 have overlapping but different transcriptional targets and there is evidence that HIF-2 is the primary driver of oncogenesis (some studies suggest that HIF-1 is antioncogenic) [46,47,48].
The role of pVHL in oxygen-dependant hypoxia-inducible factor regulated gene expression. Under conditions of normal cellular oxygen levels, hypoxia-inducible factors α-subunits (HIF1α and HIF2α) are hydroxylated by prolyl hydroxylases. Prolyl-hydroxylated HIFα is recognised by the pVHL–elongin C (ELC)–elongin B–cullin 2 (CUL2)–RBX1 (VCB–CR) E3 ubiquitin ligase complex and targeted for ubiquitylation (Ub) and proteasomal degradation. In hypoxic conditions, prolyl hydroxylases are inactive and HIFα accumulates and forms heterodimers with HIF1β which translocate to the nucleus, bind hypoxia-response elements (HREs) and induce gene transcription. The absence of VHL therefore leads to the constitutive activation of these hypoxic responsive genes (adapted from Gossage et al. (1))
The complex genotype-phenotype correlations observed in VHL disease might suggest multiple and tissue-specific pVHL functions and many HIF-dependent and HIF-independent functions have been described including regulation of extracellular matrix assembly, microtubule stability, primary cilium signalling, cell cycle progression, senescence and apoptosis and cellular metabolism (see 1, 38, 49 and reference within). In addition to HIF-1 and HIF-2 and other components of the VCBC-R complex, pVHL has been reported to interact with TP53 and absence of pVHL is associated with reduced levels of TP53 [49].
There has been considerable interest in identifying proteins, in addition to HIF-1 and HIF-2, that the VCBC-R complex might regulate. Recently, Zhang et al. [50•] undertook a screen to identify candidate proline-hydroxylation-dependent pVHL-binding partners. A candidate TSG, ZHX2 was identified from the screen and confirmed to be targeted for proteasomal degradation by pVHL in a prolyl hydroxylation-dependent manner. Further experiments demonstrated that expression of ZHX2 (Zinc fingers and homeoboxes 2) was upregulated in pVHL-deficient cRCC tumour cells and was oncogenic suggesting that ZHX2 could provide a novel target for the development of therapies for cRCC [50•].
The structure of the VCB complex has been elucidated and two coupled pVHL domains identified, an α-domain that interacts with elongin C and a β-domain that functions as the substrate docking site and binds to HIF-1a [41]. The prototypic VHL disease type 2A (p.Tyr98His) and type 2B (p.Arg167Trp/p.Arg167GLn) mutations (see Table 2) disrupt respectively the HIF-1α/β-domain and the elongin C/α-domain binding sites. In a recent study, Minervini et al. [51] used computational modelling to propose that five different interfaces of pVHL can be delineated and, using published data from 360 VHL mutations and 59 proposed pVHL interactors, they attempted to correlate the phenotypic effects of VHL missense variants with their effect on the various interactors. These findings will provide a framework for functional studies aimed at determining the molecular basis for the complex genotype-phenotype correlations observed in VHL disease and VHL-related polycythaemia disorders.
Despite the evidence for multiple roles of pVHL, regulation of HIF-1 and HIF-2 remains the best characterised and most strongly linked to tumourigenesis. Previously, it was demonstrated that it was possible to overcome the growth suppressor activity of pVHL in cRCC cells by transfecting a modified HIF-2 protein that did not bind pVHL [52]. Furthermore, the clinical utility of tyrosine kinase inhibitors which antagonise the downstream effects of activation of the hypoxic gene response pathway in metastatic RCC is well established (although progression-free survival is increased, they are not curative [53] it could be postulated that more upstream inhibition of the pathway might be more effective. Whilst it was initially thought that the HIF transcription factors were undruggable, using knowledge of the HIF-2 structure, small molecule selective HIF-2 antagonists, PT2399 and PT2385, were developed which suppressed the binding of HIF-2a to its dimerization partner ARNT/HIF-1B [54, 55••, 56••]. The inhibitors were demonstrated to suppress the growth of VHL-deficient cRCC cells in in vitro and in vivo models though some VHL-mutant tumours were resistant to treatment and prolonged treatment could lead to resistance through the acquisition of mutations in HIF-2a and HIF-1B [55••, 56••]. Subsequently, a phase 1 first-in-human study of PT2385 was reported in patients with previously treated advanced or metastatic RCC and found to be well tolerated (anaemia was the most common side effect). In 50 patients, complete response, partial response, stable disease and tumour progression were observed in 2%, 12%, 52% and 34% respectively [57•].
Management of VHL Disease
Tumour Surveillance Protocols
Large clinical studies demonstrating that the lifetime risks of retinal and cerebellar haemangioblastomas and cRCC in individuals with VHL disease exceeded 70% led to the recommendation that affected individuals and their at risk relatives should be offered annual screening from childhood [4, 6]. Whilst screening protocols may differ between centres and countries, there is clear evidence that surveillance reduces morbidity and mortality in individuals with VHL disease [58]. A recent UK national audit of centres treating patients with VHL disease (Sandford unpublished observations) showed complete compliance with a previously published screening protocol [6] though there was some variation in whether ultrasonogrophy (USS) or MRI scans are used for annual abdominal screening. Our own policy is to use annual Magnetic Resonance Imaging (MRI) screening but at a recent UK Cancer Genetics Group meeting the consensus was to start annual abdominal screening between 14 and 16 years by USS, and from age 16 years, alternate annually between MRI and USS (see CGG Spring Meeting Workshop 2019 at https://www.ukcgg.org/information-education/ukcgg-consensus-meetings/). A notable transformation in the management of VHL disease in the last three decades has been in relation to the early detection and treatment of retinal haemangioblastomas and RCC. Annual ophthalmological surveillance and early treatment of small retinal haemangioblastomas has been adopted worldwide (though optic nerve haemangioblastomas may present difficult treatment decisions) [59]. Similarly, annual renal imaging has enabled early detection of tumours and there has been worldwide acceptance of the “3-cm rule”. Small asymptomatic solid renal lesions are followed until they reach 3-cm diameter and are then treated using a nephron-sparing approach such as partial nephrectomy, radiofrequency or cryo-ablation [60, 61]. The high incidence of multicentric RCC in VHL disease means that patients may require multiple interventions but a “nephron-sparing strategy” has reduced the need for renal replacement therapy in patients with bilateral synchronous or metachronous RCC [60,61,62].
Although the need for annual surveillance programmes for patients with VHL disease is universally agreed and adopted, there is some variability in the fine details of surveillance programmes (e.g. age at which surveillance should commence, frequency of screening for CNS haemangioblastomas and screening for rare manifestations) and what follow-up should be offered to individuals with an apparently sporadic CNS haemangioblastoma and no detectable VHL mutation. It is likely that many, especially older, cases of CNS haemangioblastoma are not routinely offered VHL gene testing at all. These differences can reflect how low levels of risk are perceived (e.g. should screening start dates be based on exceptional early onset cases or a specific risk threshold—and if so, should this be 1%, 2% etc.) and, for rarer manifestations, limited data on which to base a decision. Within UK centres, there is variability in screening for pancNETs and ELSTs. Pancreatic tumours occur in ~ 10–15% of patients and previously, it has been suggested that pancNETs in VHL disease should be removed when they reached a diameter of 2–3 cm [63]. However, it is important to avoid overtreatment and to distinguish between cystadenomas (which do not require removal) and pancNETs. In a recent multicentre international study of 485 pancNETs that occurred in 273 of 2330 (11.7%) patients with VHL disease, 20% had metastatic disease [64]. Predictors of metastatic disease were a tumour diameter > 2.8 cm, high tumour growth rate and germline missense mutations at codon 167 and so the management of pancreatic lesions might be personalised according to patient-specific characteristics [64, 65].
Though the frequency of ELST in VHL disease has been reported to be as high as 11–16% in cohorts in which ELST has been screened for [66], a recent study reported a much lower frequency of 3.6%, with ELST being the initial manifestation of VHL in 32% of these cases [67]. This latter figure is more consistent with the frequency in UK centres (Sandford et al. unpublished).
A concern for many women with VHL disease is whether pregnancy might lead to a hastening of tumour growth. In our experience, this has not occurred but in the literature, there are case reports of women presenting during pregnancy with a symptomatic haemangioblastoma. A recent report from Denmark provides some reassurance regarding pregnancy-related risks as no evidence was found that pregnancy aggravated the development of VHL tumours [68]. Though this was a small study (17 women completed 30 pregnancies), it is in accordance with our own practice in which we organise baseline scans in women who are planning to become pregnant and do not perform more frequent surveillance during pregnancy if there is no evidence of tumours.
Therapeutic Options in VHL Disease
In cases of VHL disease undergoing annual surveillance, the early detection and treatment pathways for symptomatic retinal haemangioblastomas and cRCC are well established (see above). For central nervous system haemangioblastomas, the detection of an asymptomatic lesion will usually not result in surgical removal as the risks of intervention and neurological deficit need to be balanced against the possibility that the haemangioblastomas may remain asymptomatic and static or slow growing. Hence, regular monitoring will usually be initiated and surgery delayed until symptoms develop (haemangioblastomas associated with a cyst are more likely to become symptomatic). Radiosurgery has been used with some success in some centres as an alternative to neurosurgical removal [69] but the management of patients with multiple or recurrent haemangioblastomas, particularly in the spinal cord or brain stem, can be extremely challenging and the availability of effective medical approaches would be a major advance. Though there have been suggestions that the widely-used beta-blocker propranolol [70, 71] or the somatostatin analogue ocreotide [72] might provide a potential therapeutic option, there are no reports from prospective well-powered clinical trials. Tyrosine kinase inhibitors such as sorafenib, sunitnib and pazopanib have been show to extend progression-free survival in metastatic cRCC (see above) and seemingly should provide an option for systemic treatment in patients with VHL disease. In a prospective phase 2 clinical trial of pazopanib, Jonasch et al. [73•] treated 31 patients with pazopanib and observed a partial response in 42% of cases with the remaining having stable disease as the best response. The results varied by tumour site with 52% of RCC (n = 59, median shrinkage = 40.5%) and 53% of pancreatic lesions (predominantly serous cystadenomas) (responding but only 4% of CNS haemangioblastomas (n = 49, median shrinkage = 13%)). It is not clear whether long-term pazopanib treatment would alter the growth trajectory of CNS lesions and pazopanib treatment is associated with significant side effects that may require cessation of treatment. Nevertheless, this single-arm trial does suggest that systemic therapy with pazopanib might be an option for patients in whom more conventional therapies are not available or contraindicated. Furthermore, the work of Jonasch et al. [73•] will encourage further efforts to develop systemic treatments with improved efficacy and less toxicity. Small molecule HIF-2 antagonists are attracting great interest and an international trial is ongoing to investigate whether oral administration of PT2977 (a small molecule that is similar but more active than PT2385) to patients with small cRCC (< 3 cm) is associated with RCC and non-RCC tumour stabilisation or shrinkage (https://clinicaltrials.gov/ct2/show/NCT03401788).
Conclusions
Substantial progress has been made in our knowledge of the clinical and molecular features of VHL disease in the past 30 years [1, 2, 4] and the implementation of early genetic diagnosis, tumour surveillance programmes and agreed treatment approaches (e.g. 3-cm rule for renal lesions) has reduced morbidity and mortality [58]. Major challenges remain, and the development of non-toxic systemic therapies for VHL is a major goal yet to be achieved. However, there has been some progress towards this aim and the fundamental role that the VHL TSG plays in sporadic cRCC means that individuals with VHL disease should benefit from research and drug development that is primarily aimed at a much more common disorder. Another major aim must be to ensure that every individual with a pathogenic VHL variant is diagnosed early so that a surveillance programme can be initiated and their at-risk relatives identified and offered testing and surveillance. The molecular genetic tools for achieving this are available but in our experience, not all individuals who might benefit from genetic testing (e.g. patients with haemangioblastomas, PPGL etc.) are offered molecular analysis and this can result in later diagnosis in some cases. It is hoped that the mainstreaming of genomics testing in countries such as the UK and the international initiatives such as ClinGen [74] will improve both access to and the accuracy of genetic testing and further reduce avoidable morbidity and mortality from VHL disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. NatRev Cancer. 2015;15(1):55–64.
Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172–81.
Richards FM, Payne SJ, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER. Molecular analysis of de novo germline mutations in the von Hippel-Lindau disease gene. Hum Mol Genet. 1995;4(11):2139–4.
Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77(283):1151–63.
Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007;28:143–9.
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19(6):617–23.
Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science (New York, N.Y.). 1993;260(5112):1317–20.
Tabaro F, Minervini G, Sundus F, Quaglia F, Leonardi E, Piovesan D, et al. VHLdb: a database of von Hippel-Lindau protein interactors and mutations. Sci Rep. 2016;6:31128.
Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, et al. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis. 2002;28(1):57–62.
Coppin L, Grutzmacher C, Crépin M, Destailleur E, Giraud S, Cardot-Bauters C, et al. VHL mosaicism can be detected by clinical next-generation sequencing and is not restricted to patients with a mild phenotype. Eur J Hum Genet. 2014;22(9):1149–52.
Coppin L, Plouvier P, Crépin M, Jourdain AS, Ait Yahya E, Richard S, et al. Optimization of next-generation sequencing technologies for von Hippel Lindau (VHL) mosaic mutation detection and development of confirmation methods. J Mol Diagn. 2019;21:462–70.
Albanyan S, Giles RH, Gimeno EM, Silver J, Murphy J, Faghfoury H, et al. Characterization of VHL promoter variants in patients suspected of Von Hippel-Lindau disease. Eur J Med Genet. 2019;62:177–81.
• Lenglet M, Robriquet F, Schwarz K, Camps C, Couturier A, Hoogewijs D, et al. Identification of a new VHL exon and complex splicing alterations in familial erythrocytosis or von Hippel-Lindau disease. Blood. 2018;132(5):469–83. Identification of intronic mutations in individuuals without previously detected pathogenic variants.
Flores SK, Cheng Z, Jasper AM, Natori K, Okamoto T, Tanabe A, et al. A synonymous VHL variant in exon 2 confers susceptibility to familial pheochromocytoma and von Hippel-Lindau disease. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2019-00235.
Maher ER, Yates JR, Ferguson-Smith MA. Statistical analysis of the two stage mutation model in von Hippel-Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J Med Genet. 1990;27(5):311–4.
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7(1):85–90.
Foster K, Prowse A, van den Berg A, Fleming S, Hulsbeek MM, Crossey PA, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3(12):2169–73.
Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P, et al. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin Cancer Res. 2009;15(24):7582–92.
Prowse AH, Webster AR, Richards FM, et al. Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am J Hum Genet. 1997;60(4):765–71.
Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, et al. von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol. 1997;28(5):540–3.
Shankar GM, Taylor-Weiner A, Lelic N, Jones RT, Kim JC, Francis JM, et al. Sporadic hemangioblastomas are characterized by cryptic VHL inactivation. Acta Neuropathol Commun. 2014;2:167.
Webster AR, Richards FM, MacRonald FE, Moore AT, Maher ER. An analysis of phenotypic variation in the familial cancer syndrome von Hippel-Lindau disease: evidence for modifier effects. Am J Hum Genet. 1998;63(4):1025–35.
Fei SS, Mitchell AD, Heskett MB, Vocke CD, Ricketts CJ, Peto M, et al. Patient-specific factors influence somatic variation patterns in von Hippel-Lindau disease renal tumours. Nat Commun. 2016;7:11588.
Zbar B, Brauch H, Talmadge C, Linehan M. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. Nature. 1987;327(6124):721–4.
Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosom Cancer. 1998;22(3):200–9.
Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, et al. Patterns of gene expression and copy-number alterations in von-Hippel Lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 2009;69(11):4674–81.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–9.
Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463(7279):360–3.
Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–42.
Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44(7):751–9.
•• Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. Cancer Genome Atlas Research Network, Spellman PT, Rathmell WK, Linehan WM. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23(12):3698. Large-scale renal cell carcinoma cancer genomic study.
Wang SS, Gu YF, Wolff N, Stefanius K, Christie A, Dey A, et al. Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis. Proc Natl Acad Sci U S A. 2014;111(46):16538–43.
Gao W, Li W, Xiao T, Liu XS, Kaelin WG Jr. Inactivation of the PBRM1 tumor suppressor gene amplifies the HIF-response in VHL-/- clear cell renal carcinoma. Proc Natl Acad Sci U S A. 2017;114(5):1027–32.
Nargund AM, Pham CG, Dong Y, Wang PI, Osmangeyoglu HU, Xie Y, et al. The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma. Cell Rep. 2017;18(12):2893–906.
Espana-Agusti J, Warren A, Chew SK, Adams DJ, Matakidou A. Loss of PBRM1 rescues VHL dependent replication stress to promote renal carcinogenesis. Nat Commun. 2017;8(1):2026.
•• Mitchell TJ, Turajlic S, Rowan A, Nicol D, Farmery JHR, O’Brien T, et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx Renal. Cell. 2018;173(3):611–623.e17. This study provides novel insights into the evolution of clear cell renal cell carcinoma.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. https://doi.org/10.1038/20459.
Schödel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69(4):646–57.
Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–6.
Kamura T, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Pause, A. et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci U S A. 1997;94:2156–61.
Stebbins CE, Kaelin WG Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–61.
Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, et al. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 1999;96:12436–41.
Cockman ME, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 275:25733–41.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72.
Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74.
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–86.
Shen C, Beroukhim R, Schumacher SE, Zhou J, Chang M, Signoretti S, et al. Genetic and functional studies implicate HIF1α as a 14q kidney cancer suppressor gene. Cancer Discov. 2011;1(3):222–35.
Roe JS, Youn HD. The positive regulation of p53 by the tumor suppressor VHL. Cell Cycle. 2006;5(18):2054–6.
• Zhang J, Wu T, Simon J, Takada M, Saito R, Fan C, et al. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science. 2018;361(6399):290–5. https://doi.org/10.1126/science.aap8411. Identification of novel pVHL target.
Minervini G, Quaglia F, Tabaro F, Tosatto SCE. Genotype-phenotype relations of the von Hippel-Lindau tumor suppressor inferred from a large-scale analysis of disease mutations and interactors. PLoS Comput Biol. 2019;15(4):e1006478.
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1(3):237–46.
Gul A, Rini BI. Adjuvant therapy in renal cell carcinoma. Cancer. 2019. https://doi.org/10.1002/cncr.32144.
Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, et al. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. CancerRes. 2016;76(18):5491–500.
•• Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539(7627):112–7.
•• Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, et al. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. 2016;539(7627):107–11.
• Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J Clin Oncol. 2018;36(9):867–87. References 55–58 describe the discovery and potential use small molecule HIF-2 antagonist.
Maddock IR, Moran A, Maher ER, Teare MD, Norman A, Payne SJ, et al. A genetic register for von Hippel-Lindau disease. J Med Genet. 1996;33(2):120–7.
Wiley HE, Krivosic V, Gaudric A, Gorin MB, Shields C, Shields J, et al. Management of retinal hemangioblastoma in von Hippel-Lindau disease. Retina. 2019. https://doi.org/10.1097/IAE.0000000000002572.
Steinbach F, Novick AC, Zincke H, Miller DP, Williams RD, Lund G, et al. Treatment of renal-cell carcinoma in von Hippel-Lindau disease - a multicenter study. J Urol. 1995;153:1812–6.
Walther MM, Choyke PL, Glenn G, Lyne JC, Rayford W, Venzon D, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161:1475–9.
Goldfarb DA, Neumann HPH, Penn I, et al. Results of renal transplantation in patients with renal cell carcinoma in Von Hippel-Lindau disease. Transplantation. 1998;64:1726–9.
Libutti SK, Choyke PL, Bartlett DL, Vargas H, Walther M, Lubensky I, et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124(6):1153–9.
Krauss T, Ferrara AM, Links TP, Wellner U, Bancos I, Kvachenyuk A, et al. Preventive medicine of von Hippel-Lindau disease-associated pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2018;25(9):783–93.
Tirosh A, Sadowski SM, Linehan WM, Libutti SK, Patel D, Nilubol N, et al. Association of VHL genotype with pancreatic neuroendocrine tumor phenotype in patients with von Hippel-Lindau disease. JAMA Oncol. 2018;4(1):124–6.
Manski TJ, Heffner DK, Glenn GM, Patronas NJ, Pikus AT, Katz D, Lebovics R, Sledjeski K, Choyke PL, Zbar B, Linehan WM, Oldfield EH, Oldfield EH. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997;277(18):1461–1466.
Bausch B, Wellner U, Peyre M, Boedeker CC, Hes FJ, Anglani M, et al. International Endolymphatic Sac Tumor (ELST) Consortium. Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the International Endolymphatic Sac Tumor Registry. Head Neck. 2016;38(Suppl 1):E673–9.
Binderup ML, Budtz-Jørgensen E, Bisgaard ML. New von Hippel-Lindau manifestations develop at the same or decreased rates in pregnancy. Neurology. 2015;85(17):1500–3.
Liebenow B, Tatter A, Dezarn WA, Isom S, Chan MD, Tatter SB. Gamma knife stereotactic radiosurgery favorably changes the clinical course of hemangioblastoma growth in von Hippel-Lindau and sporadic patients. J Neuro-Oncol. 2019;142(3):471–8.
Albiñana V, Villar Gómez de Las HK, Serrano-Heras G, Segura T, Perona-Moratalla AB, Mota-Pérez M, et al. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis. 2015;10:118.
Shepard MJ, Bugarini A, Edwards NA, Lu J, Zhang Q, Wu T, et al. Repurposing propranolol as an antitumor agent in von Hippel-Lindau disease. J Neurosurg. 2018;1:1–9.
Sizdahkhani S, Feldman MJ, Piazza MG, Ksendzovsky A, Edwards NA, Ray-Chaudhury A, et al. Somatostatin receptor expression on von Hippel-Lindau-associated hemangioblastomas offers novel therapeutic target. Sci Rep. 2017;7:40822.
• Jonasch E, McCutcheon IE, Gombos DS, Ahrar K, Perrier ND, Liu D, et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol. 2018;19(10):1351–9. Clinical trial for tyrosine kinase inhibitor in patients with von Hippel-Lindau disease.
Rivera-Muñoz EA, Milko LV, Harrison SM, Azzariti DR, Kurtz CL, Lee K, Mester JL, Weaver MA, Currey E, Craigen W, Eng C, Funke B, Hegde M, Hershberger RE, Mao R, Steiner RD, Vincent LM, Martin CL, Plon SE, Ramos E, Rehm HL, Watson M, Berg JS ClinGen Variant Curation Expert Panel experiences and standardized processes for disease and gene-level specification of the ACMG/AMP guidelines for sequence variant interpretation. Hum Mutat 2018 Nov;39(11):1614–1622.
Acknowledgements
We apologise to all the authors whose papers we were unable to cite because of space constraints.
Funding
We thank the European Research Council (Advanced Researcher Award (EM)), NIHR (Senior Investigator Award (EM) and Cambridge NIHR Biomedical Research Centre (EM, RS)) and Cancer Research UK Cambridge Cancer Centre (EM) for research support. The University of Cambridge has received salary support in respect of EM and RS from the NHS in the East of England through the Clinical Academic Reserve.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Maher declares personal fees as a consultant to Illumina, and his role as a medical advisor to VHL Patient support groups. Dr. Sandford declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS or Department of Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of Topical Collection on Cancer Genomics
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maher, E.R., Sandford, R.N. von Hippel-Lindau Disease: an Update. Curr Genet Med Rep 7, 227–235 (2019). https://doi.org/10.1007/s40142-019-00180-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40142-019-00180-9