Abstract
Purpose of Review
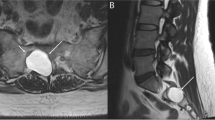
Delayed onset of communication and swallowing dysfunction due to radiation-associated neuromuscular injury is one of the most challenging clinical presentations in head and neck cancer rehabilitation. This review details the current literature and describes an evidence-based process for evaluating and treating this unique clinical entity.
Recent Findings
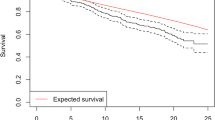
Radiation-fibrosis syndrome (RFS) is associated with lower cranial nerve palsy, dysphagia, trismus, dysarthria, dysphonia, and dyspnea. Sequelae of dysfunction can include feeding tube dependence, tracheostomy, depression, anxiety, and poor quality of life. While there is limited research evaluating rehabilitation efficacy explicitly in this population, the broader evidence base supports a multidimensional evaluation process and interventions that include compensatory approaches, skill-based training, and restorative exercises. Further evidence is forthcoming, with several ongoing randomized clinical trials exploring this topic.
Summary
Communication and swallowing dysfunction associated with RFS is debilitating, and treatment is intensive, often involving a phased approach with multiple specialties.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Stubblefield MD. Neuromuscular complications of radiation therapy. Muscle Nerve. 2017;56(6):1031–40. (This publication described basic concepts of radiation therapy (RT), the pathophysiology of radiation injury, radiation fibrosis syndrome (RFS), and the identification and evaluation of the neuromuscular late effects of radiation in cancer survivors.)
Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MW, Holsinger FC. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118(23):5793–9.
Awan MJ, Mohamed AS, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, Holsinger FC, Schwartz DL, Fuller CD, Hutcheson KA. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol. 2014;50(8):746–52.
Goldsmith T, Jacobson MC. Managing the late effects of chemoradiation on swallowing: bolstering the beginning, minding the middle, and cocreating the end. Curr Opin Otolaryngol Head Neck Surg. 2018;26(3):180–7.
•• Aggarwal P, Goepfert RP, Garden AS, Garg N, Zaveri JS, Du XL, Swartz MD, Lai SY, Fuller CD, Ferrarotto R, et al. Risk and clinical risk factors associated with late lower cranial neuropathy in long-term oropharyngeal squamous cell carcinoma survivors. JAMA Otolaryngol-Head Neck Surg. 2021;147(5):469–78. (This cohort study estimated the cumulative incidence of and identified clinical factors associated with late lower LCNP among long-term oropharyngeal squamous cell cancer survivors.)
• Dong Y, Ridge JA, Ebersole B, Li T, Lango MN, Churilla TM, Donocoff K, Bauman JR, Galloway TJ. Incidence and outcomes of radiation-induced late cranial neuropathy in 10-year survivors of head and neck cancer. Oral Oncol. 2019;95:59–64. (This retrospective review characterized the late cranial neuropathy among 10-year survivors of head and neck cancer treatment.)
Baudelet M, Van den Steen L, Tomassen P, Bonte K, Deron P, Huvenne W, Rottey S, De Neve W, Sundahl N, Van Nuffelen G, et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck. 2019;41(10):3594–603.
Kamal M, Mohamed ASR, Volpe S, Zaveri J, Barrow MP, Gunn GB, Lai SY, Ferrarotto R, Lewin JS, Rosenthal DI, et al. Radiotherapy dose-volume parameters predict videofluoroscopy-detected dysphagia per DIGEST after IMRT for oropharyngeal cancer: results of a prospective registry. Radiother Oncol. 2018;128(3):442–51.
Anderson MD, Head and Neck Cancer Symptom Working Group. Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol. 2016;118(2):304–14.
Patterson JM, McColl E, Carding PN, Wilson JA. Swallowing beyond six years post (chemo)radiotherapy for head and neck cancer; a cohort study. Oral Oncol. 2018;83:53–8.
Nilsen ML, Mady LJ, Hodges J, Wasserman-Wincko T, Johnson JT. Burden of treatment: reported outcomes in a head and neck cancer survivorship clinic. Laryngoscope. 2019;129(12):E437-e444.
•• Aggarwal P, Zaveri JS, Goepfert RP, Shi Q, Du XL, Swartz M, Gunn GB, Lai SY, Fuller CD, Hanna EY, et al. Symptom burden associated with late lower cranial neuropathy in long-term oropharyngeal cancer survivors. JAMA Otolaryngol Head Neck Surg. 2018;144(11):1066–76. (In this cross-sectional study of 889 patients, authors investigated (1) the association of late (≥ 3 months post-cancer treatment) lower cranial neuropathy (LCNP) with the severity of oncology treatment-induced symptoms and (2) functional impairment among patients treated for oropharyngeal cancers.)
•• Aggarwal P, Zaveri JS, Goepfert RP, Shi Q, Du XL, Swartz M, Lai SY, Fuller CD, Lewin JS, Piller LB, et al. Swallowing-related outcomes associated with late lower cranial neuropathy in long-term oropharyngeal cancer survivors: cross-sectional survey analysis. Head Neck. 2019;41(11):3880–94. (In this survey study, OPC survivors with late LCNP reported significantly poorer swallowing-related QOL and had a significantly higher likelihood of poor functional status. )
Hutcheson KA, Yuk M, Hubbard R, Gunn GB, Fuller CD, Lai SY, Lin H, Garden AS, Rosenthal DI, Hanna EY, et al. Delayed lower cranial neuropathy after oropharyngeal intensity-modulated radiotherapy: a cohort analysis and literature review. Head Neck. 2017;39(8):1516–23.
• Nachalon Y, Nativ-Zeltzer N, Evangelista LM, Dhar SI, Lin SJ, Shen SC, Belafsky PC. Cervical fibrosis as a predictor of dysphagia. Laryngoscope. 2021;131(3):548–52. (These cross-sectional data suggest that cervical fibrosis is associated with swallowing dysfunction.)
Anschuetz L, Shelan M, Dematté M, Schubert AD, Giger R, Elicin O. Long-term functional outcome after laryngeal cancer treatment. Radiat Oncol. 2019;14(1):101.
Kanayama N, Otozai S, Yoshii T, Toratani M, Ikawa T, Wada K, Hirata T, Morimoto M, Konishi K, Ogawa K, et al. Death unrelated to cancer and death from aspiration pneumonia after definitive radiotherapy for head and neck cancer. Radiother Oncol. 2020;151:266–72.
Nilsen ML, Belsky MA, Scheff N, Johnson JT, Zandberg DP, Skinner H, Ferris R. Late and long-term treatment-related effects and survivorship for head and neck cancer patients. Curr Treat Options Oncol. 2020;21(12):92.
• Deng J, Wulff-Burchfield EM, Murphy BA. Late soft tissue complications of head and neck cancer therapy: lymphedema and fibrosis. J Natl Cancer Inst Monogr. 2019;2019(53):lgz005. This review discussed profound long-term symptom burden, loss of critical functions, and altered quality of life as a result of lymphedema and fibrosis; the authors also reviewed pathobiology, clinical manifestations, and future directions for research related to lymphedema and fibrosis.
Langmore SE, McCulloch TM, Krisciunas GP, Lazarus CL, Van Daele DJ, Pauloski BR, Rybin D, Doros G. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: a randomized clinical trial. Head Neck. 2016;38 Suppl 1(Suppl 1):E1221-1231.
Calman KC. Quality of life in cancer patients–an hypothesis. J Med Ethics. 1984;10(3):124–7.
List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–9.
Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–20.
Rosenthal DI, Chambers MS, Mendoza TR, Asper JA, Kies MS, Weber RS, Garden AS, Ang KK, Wang XS, Cleeland CS. The reliability and validity of the M. D. Anderson Symptom Inventory (MDASI-HN) as a measure of symptom burden in the head and neck cancer (HNC) patient population. J Clin Oncol. 2005;23(16_suppl):8097–8097.
Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14(4):198–205.
Deng J, Ridner SH, Wells N, Dietrich MS, Murphy BA. Development and preliminary testing of head and neck cancer related external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2015;19(1):75–80.
Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):153–8.
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90–2.
Starmer HM, Drinnan M, Bhabra M, Watson LJ, Patterson J. Development and reliability of the revised Patterson Edema Scale. Clin Otolaryngol. 2021;46(4):752–7.
Logemann J. Evaluation and treatment of swallowing disorders. In: Logemann J, editor. 2nd edn. Austin, TX: Pro-Ed; 1998.
Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia. 2014;29(2):223–33.
Simcock R, Simo R. Follow-up and survivorship in head and neck cancer. Clin Oncol (R Coll Radiol). 2016;28(7):451–8.
Soni RS, Ebersole B, Jamal N. Does even low-grade dysphonia warrant voice center referral? J Voice. 2017;31(6):753–6.
Woo P, Colton R, Casper J, Brewer D. Diagnostic value of stroboscopic examination in hoarse patients. J Voice. 1991;5(3):231–8.
Woo P, Parasher AK, Isseroff T, Richards A, Sivak M. Analysis of laryngoscopic features in patients with unilateral vocal fold paresis. Laryngoscope. 2016;126(8):1831–6.
Mallur PS, Rosen CA. Vocal fold injection: review of indications, techniques, and materials for augmentation. Clin Exp Otorhinolaryngol. 2010;3(4):177–82.
Woo P. Stroboscopy and high-speed imaging of the vocal function. 2nd ed. San Diego, CA: Plural Publishing; 2022.
Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment-MBSImp: establishing a standard. Dysphagia. 2008;23(4):392–405.
Stoeckli SJ, Huisman TA, Seifert B, Martin-Harris BJ. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18(1):53–7.
Stokely SL, Molfenter SM, Steele CM. Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia. 2014;29(1):78–82.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8.
Hutcheson KA, Barrow MP, Barringer DA, Knott JK, Lin HY, Weber RS, Fuller CD, Lai SY, Alvarez CP, Raut J, Lazarus CL, May A, Patterson J, Roe JW, Starmer HM, Lewin JS. Dynamic imaging grade of swallowing toxicity (DIGEST): scale development and validation. Cancer 2017;123(1):62–70.
Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res. 1994;37(2):314–25.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. 2014;57(3):768–78.
Thompson TZ, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, Sokolove RL, Taylor BK, Tuck JM, Pearson WG Jr. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014;(87):51476.
Starmer HM, Arrese L, Langmore S, Ma Y, Murray J, Patterson J, Pisegna J, Roe J, Tabor-Gray L, Hutcheson K. Adaptation and validation of the dynamic imaging grade of swallowing toxicity for flexible endoscopic evaluation of swallowing: DIGEST-FEES. J Speech Lang Hear Res. 2021;64(6):1802–10.
Donnan EN, Pandolfino JE. EndoFLIP in the esophagus: assessing sphincter function, wall stiffness, and motility to guide treatment. Gastroenterol Clin North Am. 2020;49(3):427–35.
Lee TH, Lee JS, Hong SJ, Jeon SR, Kim WJ, Kim HG, Cho JY, Kim JO, Cho JH, Kim MY, et al. Impedance analysis using high-resolution impedance manometry facilitates assessment of pharyngeal residue in patients with oropharyngeal dysphagia. J Neurogastroenterol Motil. 2014;20(3):362–70.
Omari TI, Ferris L, Dejaeger E, Tack JF, Vanbeckevoort D, Rommel N. Upper esophageal sphincter impedance as a marker of sphincter opening diameter. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G909–13.
Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia. 2014;29(1):2–16.
• Van Daele DJ, Langmore SE, Krisciunas GP, Lazarus CL, Pauloski BR, McCulloch TM, Gramigna GD, Messing BP, Wagner CW, Mott SL. The impact of time after radiation treatment on dysphagia in patients with head and neck cancer enrolled in a swallowing therapy program. Head Neck. 2019;41(3):606–14. (Authors reported beginning a swallowing therapy program within 1 year of RT demonstrated more consistent improvement in QOL and diet performance compared to later periods.)
A Joint Center for Health Systems Innovation (www.ariadnelabs.org) and Dana-Farber Cancer Institute. Serious illness conversation guide. 2017. Licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0.
Strand EA. Treatment of dysarthria: support by evidence-based research and expert opinion. Perspect Neurophysiol Neurogenic Speech Lang Disord. 2002;12(4):3–4.
McCabe D, Ashford J, Wheeler-Hegland K, Frymark T, Mullen R, Musson N, Hammond CS, Schooling T. Evidence-based systematic review: oropharyngeal dysphagia behavioral treatments. Part IV–impact of dysphagia treatment on individuals’ postcancer treatments. J Rehabil Res Dev. 2009;46(2):205–14.
Oh D-H, Park H-S, Kim G-E. Effects of the chin-tuck maneuver on anatomical changes and angles during swallowing: a systematic review. J Korean Dysphagia Soc. 2022;12(1):1–13.
Benjapornlert P, Kagaya H, Inamoto Y, Mizokoshi E, Shibata S, Saitoh E. The effect of reclining position on swallowing function in stroke patients with dysphagia. J Oral Rehabil. 2020;47(9):1120–8.
Umeda Y, Mikushi S, Amagasa T, Omura K, Uematsu H. Effect of the reclining position in patients after oral tumor surgery. J Med Dent Sci. 2011;58(2):69–77.
Tirado Y, Lewin JS, Hutcheson KA, Kupferman ME. Office-based injection laryngoplasty in the irradiated larynx. Laryngoscope. 2010;120(4):703–6.
Zuniga S, Ebersole B, Jamal N. Improved swallow outcomes after injection laryngoplasty in unilateral vocal fold immobility. Ear Nose Throat J. 2018;97(8):250–6.
O’Dell K, Hubanks J. In-office injection pharyngoplasty for velopharyngeal insufficiency after oropharyngeal cancer treatment. Laryngoscope. 2019;129(12):2740–3.
Asfar MM, Hutcheson KA, Won AM. Prosthetic rehabilitation with palatal lift/augmentation in a patient with neurologic/motor deficit due to cancer therapy for chondrosarcoma. J Prosthodont. 2019;28(3):234–8.
Nomoto A, Ohno T, Kunieda K, Kanazawa H, Shigematsu T, Hojo K, Shimizu A, Minakuchi S, Fujishima I. Evaluation of a palatal lift prosthesis with a flexible lift in a lower cranial nerve palsy patient with dysphagia using high-resolution manometry: a case report. J Prosthodont Res. 2021;65(4):573–6.
Bou C, Liang Fat AS, de Mones Del Pujol E, Plaire V, Naveau A. A new membrane obturator prosthesis concept for soft palate defects. Int J Prosthodont. 2018;31(6):584–6.
Huckabee M-L, Lamvik-Gozdzikowska K. Reconsidering rehabilitation for neurogenic dysphagia: strengthening skill in swallowing. Curr Phys Med Rehabil Rep. 2018;6(3):186–91.
Denk DM, Kaider A. Videoendoscopic biofeedback: a simple method to improve the efficacy of swallowing rehabilitation of patients after head and neck surgery. ORL J Otorhinolaryngol Relat Spec. 1997;59(2):100–5.
Simson G, Govender R. Fiberoptic endoscopic evaluation of swallowing as a tool to facilitate dysphagia rehabilitation following a salvage hemi-glossectomy: case report. Adv Commun Swallowing. 2022;25:61–71.
Martin-Harris B, McFarland D, Hill EG, Strange CB, Focht KL, Wan Z, Blair J, McGrattan K. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil. 2015;96(5):885–93.
Crary MA, Carnaby GD, LaGorio LA, Carvajal PJ. Functional and physiological outcomes from an exercise-based dysphagia therapy: a pilot investigation of the McNeill Dysphagia Therapy Program. Arch Phys Med Rehabil. 2012;93(7):1173–8.
Carnaby-Mann GD, Crary MA. McNeill dysphagia therapy program: a case-control study. Arch Phys Med Rehabil. 2010;91(5):743–9.
Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–65.
Van den Steen L, Baudelet M, Tomassen P, Bonte K, De Bodt M, Van Nuffelen G. Effect of tongue-strengthening exercises on tongue strength and swallowing-related parameters in chronic radiation-associated dysphagia. Head Neck. 2020;42(9):2298–307.
Moon JH, Hong DG, Kim KH, Park YA, Hahm SC, Kim SJ, Won YS, Cho HY. Effects of lingual strength training on lingual strength and articulator function in stroke patients with dysarthria. J Phys Ther Sci. 2017;29(7):1201–4.
McKenna VS, Zhang B, Haines MB, Kelchner LN. A systematic review of isometric lingual strength-training programs in adults with and without dysphagia. Am J Speech Lang Pathol. 2017;26(2):524–39.
• Lin CJ, Lee YS, Hsu CF, Liu SJ, Li JY, Ho YL, Chen HH. Effects of tongue strengthening exercises on tongue muscle strength: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2022;12(1):10438. (In this systematic review and meta-analysis, authors proport positive evidence that tongue strengthening exercise may be beneficial in improving tongue strength and could be applied for adults, especially healthy older adults.)
Laciuga H, Rosenbek JC, Davenport PW, Sapienza CM. Functional outcomes associated with expiratory muscle strength training: narrative review. J Rehabil Res Dev. 2014;51(4):535–46.
Hutcheson KA, Hammer MJ, Rosen SP, Jones CA, McCulloch TM. Expiratory muscle strength training evaluated with simultaneous high-resolution manometry and electromyography. Laryngoscope. 2017;127(4):797–804.
Hutcheson KA, Barrow MP, Plowman EK, Lai SY, Fuller CD, Barringer DA, Eapen G, Wang Y, Hubbard R, Jimenez SK, et al. Expiratory muscle strength training for radiation-associated aspiration after head and neck cancer: a case series. Laryngoscope. 2018;128(5):1044–51.
Krisciunas GP, Vakharia A, Lazarus C, Taborda SG, Martino R, Hutcheson K, McCulloch T, Langmore SE. Application of manual therapy for dysphagia in head and neck cancer patients: a preliminary national survey of treatment trends and adverse events. Glob Adv Health Med. 2019;8:2164956119844151.
Hutcheson K, McMillan H, Warneke C, Porsche C, Savage K, Buoy S, Wang J, Woodman K, Lai S, Fuller C. Manual therapy for fibrosis-related late effect dysphagia in head and neck cancer survivors: the pilot MANTLE trial. BMJ Open. 2021;11(8):e047830.
Rodríguez-Sanz J, Malo-Urriés M, Corral-de-Toro J, López-de-Celis C, Lucha-López MO, Tricás-Moreno JM, Lorente AI, Hidalgo-García C. Does the addition of manual therapy approach to a cervical exercise program improve clinical outcomes for patients with chronic neck pain in short- and mid-term? A randomized controlled trial. Int J Environ Res Public Health. 2020;17(18):6601.
McMillan H, Barbon CEA, Cardoso R, Sedory A, Buoy S, Porsche C, Savage K, Mayo L, Hutcheson KA. Manual therapy for patients with radiation-associated trismus after head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2022;148(5):418–25.
Mathieson L, Hirani SP, Epstein R, Baken RJ, Wood G, Rubin JS. Laryngeal manual therapy: a preliminary study to examine its treatment effects in the management of muscle tension dysphonia. J Voice. 2009;23(3):353–66.
van Gogh CD, Verdonck-de Leeuw IM, Boon-Kamma BA, Rinkel RN, de Bruin MD, Langendijk JA, Kuik DJ, Mahieu HF. The efficacy of voice therapy in patients after treatment for early glottic carcinoma. Cancer. 2006;106(1):95–105.
• Millgård M, Tuomi L. Voice quality in laryngeal cancer patients: a randomized controlled study of the effect of voice rehabilitation. J Voice. 2020;34(3):486.e413-486.e422. (In this randomized control trial, authors reported voice rehabilitation for irradiated laryngeal cancer patients may have positive effects on voice quality up to 24 months post-radiotherapy.)
Angadi V, Dressler E, Kudrimoti M, Valentino J, Aouad R, Gal T, Stemple J. Efficacy of voice therapy in improving vocal function in adults irradiated for laryngeal cancers: a pilot study. J Voice. 2020;34(6):962.e969-962.e918.
Lee JS, Kim JP, Ryu JS, Woo SH. Effect of wound massage on neck discomfort and voice changes after thyroidectomy. Surgery. 2018;164(5):965–71.
Hutcheson KA, Nurgalieva Z, Zhao H, Gunn GB, Giordano SH, Bhayani MK, Lewin JS, Lewis CM. Two-year prevalence of dysphagia and related outcomes in head and neck cancer survivors: an updated SEER-Medicare analysis. Head Neck. 2019;41(2):479–87.
Randall DR, Evangelista LM, Kuhn MA, Belafsky PC. Improved symptomatic, functional, and fluoroscopic outcomes following serial “series of three” double-balloon dilation for cricopharyngeus muscle dysfunction. J Otolaryngol Head Neck Surg. 2018;47(1):35.
Wu PI, Szczesniak MM, Maclean J, Graham PH, Quon H, Choo L, Cook IJ. Endoscopic dilatation improves long-term dysphagia following head and neck cancer therapies: a randomized control trial. Dis Esophagus. 2019;32(6):doy087.
Koshkareva Y, Gaughan JP, Soliman AM. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol. 2007;116(3):206–10.
Moran K ML, Soliman A. Management and functional implications of supraglottic stenosis in patients with radiation-associated dysphagia. In: American Speech Language Hearing Association Annual Convention Washington D.C., United States; 2021.
Massonet H, Goeleven A, Van den Steen L, Vergauwen A, Baudelet M, Van Haesendonck G, Vanderveken O, Bollen H, van der Molen L, Duprez F, et al. Home-based intensive treatment of chronic radiation-associated dysphagia in head and neck cancer survivors (HIT-CRAD trial). Trials. 2022;23(1):893.
Murphy BA, Dietrich MS, Wells N, Dwyer K, Ridner SH, Silver HJ, Gilbert J, Chung CH, Cmelak A, Burkey B, et al. Reliability and validity of the Vanderbilt head and neck symptom survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck. 2010;32(1):26–37.
Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, Goepfert H. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–6.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114(9):1549–56.
Gartner-Schmidt JL, Shembel AC, Zullo TG, Rosen CA. Development and validation of the dyspnea index (DI): a severity index for upper airway-related dyspnea. J Voice. 2014;28(6):775–82.
Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The communicative participation item bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res. 2013;56(4):1190–208.
Deng J, Dietrich MS, Niermann KJ, Sinard RJ, Cmelak AJ, Ridner SH, Gilbert J, Murphy BA. Refinement and validation of the head and neck lymphedema and fibrosis symptom inventory. Int J Radiat Oncol Biol Phys. 2021;109(3):747–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Hutcheson has received funding outside of submitted work from the National Institute of Health/National Cancer Institute, the National Institute of Health/National Institute of Dental and Craniofacial Research, the Patient-Centered Outcomes Research Institute, the Department of Defense and Atos Medical. Barbara Ebersole and Holly McMillan have no potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies of human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebersole, B.M., McMillan, H. & Hutcheson, K. Evaluation and Management of Speech and Swallowing Issues in RFS. Curr Phys Med Rehabil Rep 11, 93–104 (2023). https://doi.org/10.1007/s40141-023-00388-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-023-00388-5