Abstract
Introduction
Lumbar degenerative disease and the accompanying pain and dysfunction affect a significant number of patients in the USA and around the world. As surgery and innovation are moving towards minimally invasive treatments, this study looks to explore interspinous fixation as a standalone posterior approach to treat lumbar degenerative disc disease in the presence of neurogenic claudication and spinal stenosis.
Methods
This study was approved by an institutional review board (IRB) and is actively enrolling in a single-arm, multicenter, prospective, open-label fashion. Patients are followed with reporting at 3 months, and 12 months for primary endpoint analysis of efficacy and safety based on improved composite endpoints relative to baseline, with success defined as greater than 20 mm back pain reduction in Visual Analog Scale 100 mm (VAS) while standing or walking, greater than 20 mm leg pain reduction in VAS while standing or walking, Zurich Claudication Questionnaire (ZCQ) improvement of 0.5 or greater in two or three domains, Oswestry Disability Index (ODI) improvement of a least 10 points and no reoperations or revisions at the index level(s). Secondary endpoints included a multidimensional assessment in the Patient-Reported Outcomes Measurement Information System (PROMIS) 29 v2.1 and Patient Global Impression of Change (PGIC).
Results
In this interim 3-month analysis, 82% of patients reported they were improved from the procedure, while 65% of patients demonstrated clinical meaningful improvement in their pain and function, as defined by the VAS, ODI, and ZCQ. There was only one adverse event and no complications were identified at last clinic research follow-up visit.
Conclusions
This interim analysis of the first 20% of the enrolled patients out to 3 months was to determine safety of the procedure and report on adverse events, acknowledging the heterogeneity of surgical specialty. Further follow-up and greater numbers are needed as the study is ongoing.
Trial Registration
ClinicalTrials.gov identifier, NCT05504499.
Similar content being viewed by others
The treatment of lumbar spinal stenosis has a large unmet treatment need that bridges the gap between conservative measures and invasive surgical procedures. |
This study was conducted to determine the utility of using interspinous fusion devices as a standalone therapy for the treatment of lumbar spinal stenosis. |
This publication demonstrates that the use of this novel interspinous fusion device by the interventional pain community is both effective and safe at 3 months follow-up. |
Introduction
The landscape of spine surgery is changing. As did the acknowledgement of minimally invasive techniques to improve safety and at least maintain efficacy of treatments for patients in the cardiac space, the renaissance is also occurring in the spine.
Degenerative disc disease is a common condition of the aging spine, and may contribute to a variety of painful symptoms, including radiculopathy, neurogenic claudication, and back pain. There are a number of mechanical sequelae that result from these degenerative processes of the intervertebral disc, which may manifest in the anterior, middle, and posterior columns of the spine. Symptoms of degenerative disc disease can be successfully managed on the spectrum of treatment options, from conservative measures such as physical therapy and regional injection treatments, to more intensive measures such as invasive surgical decompression and/or fusion options [1, 2].
The space in between was created to minimize surgical complications and to avoid protracted recovery periods by looking for minimally invasive options, including interspinous spacers (ISS) or percutaneous image-guided lumbar decompression, with debulking of the ligamentum flavum, with excellent results represented in multiple multicenter, prospective, randomized comparative studies, with follow-up out to 2–5 years [3, 4]. These minimally invasive options bridge the gap between conservative measures and more invasive larger open surgical procedures. This can potentially deliver options to those patients who are not traditional surgical candidates secondary to medical comorbidities, or for those wishing to avoid a more invasive option.
Determining approach is critical and patient candidacy paramount in selecting the right patient for the right therapy. Direct comparison of 2-year results of ISS and decompressive laminectomies found ISS offers a less invasive treatment that reduces the potential for comorbidities, necessity for future operations, and is less disruptive to the spinal anatomy providing greater options for future surgical interventions with equivalent clinical outcomes [4]. The landmark multicenter SPORT trial compared decompressive laminectomy to conservative non-operative care in patients with spinal stenosis with neurogenic claudication and found that the surgical group had significantly greater improvement in pain and function at 4-year follow-up [5]. Further, a meta-analysis of lumbar fusions for degenerative diseases looked at patient reported outcomes from 65 studies including disability, pain scores, and patient satisfaction. Fusion has been shown to be evidenced for spondylolisthesis, and patients who were randomly assigned to fusion care were four times as likely to be satisfied, attained 34% greater pain relief, and saw a 40% improvement of preoperative disability when compared to those who received non-operative care [2]. Evidence for fusion for stenosis without spondylolisthesis is limited in this meta-analysis; however, this did not distinguish among fusion approaches.
Fusion did provide greater relief than non-operative care in patients with chronic low back pain without clinically significant stenosis or spondylolisthesis [2]. Five randomized control trials reported results with fusion between 16% and 18% improved in terms of back and leg pain as compared to non-operative care [2]. Anterior interbody fusion (ABF) and posterolateral fusion with pedicle screws (PLF) in patients with discogenic low back pain resulted in a significant decrease in Visual Analog Scale 100 mm (VAS) pain scores, with greatest relief following ABF, compared to conservative treatment [6]. To evaluate the use of a standalone interspinous fixation device to offer a minimally invasive option, but also increase the patients served by allowing for fixation in patients that had developed increased spondylolisthesis or micro instability with facet joint fluid, this device may offer a solution. Biomechanically, interspinous fusion has been demonstrated to deliver immediate flexion–extension balance and provide effective stabilization for arthrodesis while preserving motion [7, 8]. Advantages include small skin incisions, minimally invasive, minimal muscle dissection, shorter operative times, and favorable efficacy [9]. The use of interspinous fusion (ISF) in solitary as a treatment was performed by Postacchini et al. who demonstrated in a prospective study that a standalone ISF, with minimally invasive decompression in stenotic patients showing degenerative spondylolisthesis, provided fusion and highly significant improvement in all outcome measures at a 2-year follow-up [10].
This study will evaluate the effectiveness and safety of the use of Aurora Spine ZIP™ MIS Interspinous Fusion System and bone graft material in single or two-level fusion in patients with chronic low back pain that present with degenerative disc disease (DDD) with concurrent neurogenic claudication. Devices included in this study are the Aurora Spine ZIP™ (Carlsbad, CA) MIS Interspinous Fusion System and bone graft material. The device is a bilateral locking plate system which attaches to the spinous processes of the posterior noncervical spine (T1–S1) from an interlaminar approach. The implants have superior and inferior spinous process articulations and a central bone graft chamber. Specific to this interspinous fusion system are certain biomechanical characteristics such as prevention of extension by acting as a rigid block in the interspinous space. Its rigid structure immobilizes the posterior elements including the lamina and spinous processes reducing motion in all three spinal columns for both flexion and extension. In conjunction with autologous bone grafting via surgical decortication and the use of exogenous allograft the device can lead to bony fusion with rigid interlaminar fixation, as well as indirect decompression.
The Aurora Spine ZIP™ is used to treat DDD (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (i.e., fracture or dislocation), and/or tumor. The studied indication is lumbar degenerative disease resulting in back pain with lower extremity symptoms and neurogenic claudication. The clinical benefits of interspinous devices have been described in previous studies based on certain biomechanical characteristics such as prevention of extension by acting as a rigid block in the interspinous space. The Zip device is a true fusion device with a rigid structure designed to immobilize the posterior elements including the lamina and spinous processes in all planes of motion, as well as to reduce motion in all three spinal columns in both flexion and extension. In conjunction with autologous bone grafting via surgical decortication and the use of exogenous allograft, we anticipate the device to lead to bony fusion of the posterior column at similar rates as pedicle screw fixation. Future data will evaluate the rate of fusion resulting from rigid interlaminar fixation with the Zip device.
Methods
Designs and Sites
This clinical investigation is a prospective, observational, open-label, non-randomized, multicenter study. IRB approval was provided by Western IRB (IRB #20211168). It is designed to collect clinical follow-up data on patients undergoing interspinous interlaminar fusion with bone graft performed on an ambulatory basis by interventional pain physicians, orthopedic, and neurosurgeons. All patient data collected were de-identified to provide patient data confidentiality and compliance with the Declaration of Helsinki. The protocol and IRB were each approved by the local governing entities of each involved institution. All enrolled subjects provided voluntary written informed consent to participate. Subjects were allowed to ask questions and were given a copy of the informed consent.
Patient Population
Patients were enrolled in the study if they were at least 18 years of age, have at least 1–2 symptomatic lumbar degenerative disc disease at adjacent levels, from T1 to S1, with or without grade I spondylolisthesis, MRI with at least mild to moderate spinal stenosis at the index level, non-operative treatment for at least 3 months, ZCQ physical function, greater or equal to 2.0 at baseline as assessment, presence of neurogenic claudication, baseline VAS of greater than 50 mm on a 100-mm scale. Baseline characteristics are summarized in Table 2. Key exclusion criteria included greater than a grade II spondylolisthesis on flexion and extension films with 3-mm instability or had previous lumbar spine surgery. Subjects were recruited from patients that presented to participating sites who met all of the inclusion and none of the exclusion criteria per protocol.
Interventions
After appropriate enrollment, as defined by the research protocol, the patent underwent the interspinous fixation (see anteroposterior radiograph in Fig. 1). It has been previously defined by Falowski et al. in a retrospective study demonstrating safety and efficacy [11]. Of note, prior to the procedure, all patients received an authorization through their commercial or government payer insurance prior to completion of surgery.
Follow-up
Patients enrolled had appropriate imaging reviewed and failure of conservative therapy as defined in the protocol per principal investigator. The medical monitor served to approve all patients enrolled and active. Patients were identified and enrolled, implanted, and followed immediately postoperatively as per standard of care by site, and scheduled visits occurred at month 1, 3, 6, and 12. Enrollment goal was set at 100 patients, with potential follow-up out to 60 months.
Endpoints
Baseline demographic information and procedural detail were captured.
Primary endpoints are to evaluate efficacy and safety at 3 and 12 months based on improved composite endpoints relative to baseline, with success defined as greater than 20 mm pain reduction in Visual Analog Scale 100 mm (VAS) Back while standing or walking, greater than 20 mm pain reduction in VAS Leg while standing or walking, Zurich Claudication Questionnaire (ZCQ) improvement of 0.5 or greater in two or three domains, ODI improvement of at least 10 points and no reoperations or revisions at the index level(s).
The Oswestry Disability Index (ODI) [12] is used to measure a patient’s permanent functional disability and is considered the gold standard of low back functional outcome tools. This questionnaire was designed to provide information as to how a patient’s back or leg pain affects their ability to manage in everyday life. For each of the 10 sections the total possible score is 5. The score is a percentage calculated by the total scored divided by 50 (total possible score) × 100. The interpretation of the scores is described in Table 1.
The Zurich Claudication Questionnaire (ZCQ) is a self-administered measure to evaluate symptom severity, physical function, and surgery satisfaction in lumbar spinal stenosis (LSS). The ZCQ quantifies severity of symptoms, physical function characteristics, and patient’s satisfaction after treatment. The scale relates to symptoms over the past month. There are 12 questions related to Symptom severity scale (questions I–VII, possible range of score is 1 to 5) and Physical function scale (questions VIII–XII, possible range of score is 1 to 4) and a further six questions to measure treatment outcome. The result is expressed as a percentage of the maximum possible score, which increases with worsening disability.
Secondary endpoints included a multidimensional pain functional assessment as a Patient Reported Outcome Measurement Information System (PROMIS) 29 v2.1, Pain Impact Score (calculated from the PROMIS 29), opioid consumption related to study related pain, health care consumption, and imaging analysis at 12 months, and global impression of change relative to baseline at 3 and 12 months (PGIC).
The PROMIS 29 is a validated 29-item profile instrument that assesses eight universal domains (not disease-specific): physical function, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles and activities, pain interference, and pain intensity [13,14,15,16,17,18]. The first seven domains are assessed with four questions each; pain intensity is measured with a single 11-point numeric rating scale (NRS) from 0 (no pain) to 10 (worst imaginable pain). High scores represent more of the domain being measured. Thus, on symptom-oriented (negatively worded) domains of PROMIS 29 (anxiety, depression, fatigue, pain interference, and sleep disturbance), higher scores represent worse symptomatology. On the function-oriented (positively worded) domains (physical functioning and social role) higher scores represent better functioning. The Pain Impact Score (PIS) is a derivative of the PROMIS 29 that ranges from 8 (low impact) to 50 (high impact). The PIS is calculated by adding the raw scores for pain intensity [0–10] and pain interference [4–20] along with the inverted raw score for physical function [4–20].
Statistical Analysis
For patient self-reported assessments including the VAS, ODI, PROMIS 29, PGIC, and quantitative outcomes, paired t test was performed. Analysis of variance (ANOVA) regression analysis was utilized to identify and verify the correlations reported. The data analysis was performed utilizing IBM SPSS statistics.
Results
Eight centers participated in the study, spanning neurosurgery and interventional pain management specialties, with enrollment that began on March 21, 2021 and is currently actively enrolling.
Patient Population
Baseline characteristics are summarized in Table 2.
Patient Activity
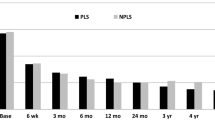
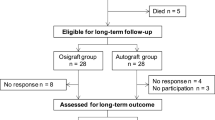
This data represents an interim analysis of safety and efficacy at 3 months in an ongoing study. Future analysis will report on the complete data set of this multicenter, prospective, single-arm study. Currently there are 54 active patients, 11 terminated patients and 32 implanted. Nineteen represent nearly a fifth of the goal enrollment. See Fig. 2. This represents a single-arm, prospective, multicenter study with follow-up anticipated with primary endpoint at 3 months and 12 months, with follow-up out to 5 years.
Surgical Information
Surgical data was acquired and verified. Please see Table 3.
Clinical Outcomes
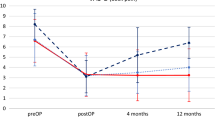
Patient’s pain intensity was measured using a VAS 100 mm, documented from baseline as a difference to document change for back and for leg (Fig. 3), with success defined as greater than 20 mm reduction while standing and walking. Success according to ZCQ was defined as as an improvement from baseline of 0.5 or greater in two or three domains, while the ODI improvement of at least 10 points and no reoperations or revisions at the index level(s). Figure 4 shows the ODI improvement results. Table 4 summarizes the outcome measures.
Secondary endpoints included a multimodal assessment including a PROMIS 29 v2.1 and PGIC investigating patient satisfaction and global impression of change (Tables 5, 6).
Safety Analysis
Adverse events were monitored throughout the study activity, including worsening pain, infection, hardware malfunction, and bleeding (Table 7). All AEs were recorded and the likelihood of relation to the treatment procedure or device. One patient had worsening pain after surgery, which resolved completely with no interventions required.
Discussion
This data represents the first prospectively acquired data set for use of a standalone fixation device for the treatment of degenerative disc disease in the presence of neurogenic claudication and symptomatic spinal stenosis. This study is active and enrolling, with the goal of 100 patients at 3 months and 12 months, and planned follow-up to 5 years. The data presented an interim analysis at 3 months to evaluate safety and efficacy.
The interspinous fusion therapy as a standalone therapy has never been studied in a prospective multicenter fashion. The Coflex is an interlaminar device approved in the lumbar spine from L1 to L5 and includes a decompression before stabilization with the device. It was studied in a multicenter randomized control trial in comparison to decompression with pedicle screws and represented the Coflex as a viable alternative to pedicle screw fixation to treat lumbar spinal stenosis when combined with decompression [19]. The Aurora device does not require direct decompression.
The PrimaLOK device was studied retrospectively in 53 patients to treat spinal disorders, including stenosis, herniated disc, degenerative disc disease, and spondylolisthesis, with a 22-month follow-up on average with pain index score rating from 7.17 to 4.48, suggesting that the use of an independent interspinous fusion system could provide similar results to a pedicle screw instrumentation or interbody cage [20]. This study did not represent a standalone system, unlike the Zip device. These were reviewed in a MIS technology review [21].
Spinal stenosis in the presence of neurogenic claudication can be treated with many treatments, including surgical decompression with laminectomy, with or without fusion, epidural injection, percutaneous image guided lumbar decompression, or interspinous spacer, to name a few. The interspinous spacer (ISS) is different than the interspinous fusion (ISF), as this is an indirect decompression strategy that also offers stabilization by fusion. This was discussed with the patient during the surgical discussion.
Regarding patient safety, there was one adverse event reported, with none that were device or surgery related. Given that this procedure was performed by both neurosurgeons and pain physicians, with the majority being performed by the pain physicians, this data demonstrates the safety of the procedure at 3 months.
Pain intensity reduction, functional improvements, and patient satisfaction demonstrate benefit with the procedure from baseline. Indeed, 82% of the patients reported they were improved from the procedure. Nearly 65% patients achieved the benchmark of treatment success, and although only 3 months in, which clearly suggests immediate improvement from the procedure. All but three patients were self-reported improved by the procedure, while functional improvement was noted for 65% patients with improvement in ZCQ for symptoms and function. Functional improvement, as measured by ODI improved by 17, on average.
As stated, the PROMIS 29 v2.1 is a multidimensional tool with domains of pain intensity, function, sleep, and the psychological effects of dysfunction. Pope et al. reported on patients presenting to spine and pain centers across the USA, benchmarking typical scores across the PROMIS 29 v2.1, and typical of the population represented here [22]. Higher scores indicate more disability for negatively worded instruments, whereas higher scores demonstrate improvement for positively worded instruments. There was a mean improvement from baseline for the PROMIS 29, with statistical significance, for all but anxiety and depression. Overall, all but three patients self-reported satisfaction with the procedure, measured by the PGIC.
Limitations
Limitations to the study include being a single-arm, prospective study investigating the safety and efficacy of a device for spinous process fixation to treat degenerative disc disease in the face of neurogenic claudication and spinal stenosis. It is not a randomized study. Further, the patient cohort was limited to those approved for the procedure.
Conclusions
This interim data analysis of approximately a fifth of the patient recruitment goal serves as a planned safety analysis of the procedure. As most surgical complications occur within the first 90 days, our analysis reveals that the first consecutive 17 patients enrolled in this study show no device-related complications, suggesting safety in the hands of both neurosurgeons and interventional pain physicians. Further, pain intensity, function, sleep, anxiety, fatigue, pain interference, physical function, and social participation also demonstrated improvement.
References
Deer TR, Grider JS, Pope JE, et al. The MIST guidelines: the lumbar spinal stenosis consensus group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3):250–74.
Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Vol. 80, Clinical Neurosurgery. Oxford University Press; 2017. p. 701–15. www.neurosurgery-online.com. Accessed Dec 21, 2020.
Nunley PD, Patel VV, Gorndorff D, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017;12:1409–17.
Lauryssen C, Jackson RJ, Baron JM, et al. Stand-alone interspinous spacer versus decompressive laminectomy for treatment of lumbar spinal stenosis. Expert Rev Med Devices. 2015;12(6):763–9.
Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2010;35(14):1329–38.
Ohtori S, Koshi T, Yamashita M, et al. Surgical versus nonsurgical treatment of selected patients with discogenic low back pain: a small-sized randomized trial. Spine (Phila Pa 1976). 2011;36(5):347–54.
Gonzalez-Blohm SA, Doulgeris JJ, Aghayev K, Lee WE, Volkov A, Vrionis FD. Biomechanical analysis of an interspinous fusion device as a stand-alone and as supplemental fixation to posterior expandable interbody cages in the lumbar spine: laboratory investigation. J Neurosurg Spine. 2014;20(2):209–19. https://doi.org/10.3171/2013.10.SPINE13612.
Karahalios DG, Musacchio MJ. Lumbar interspinous devices: fusion and motion sparing. In: Holly LT, Anderson PA, editors. Essentials of spinal stabilization. Springer; 2017. p. 321–34. https://doi.org/10.1007/978-3-319-59713-3_25.
Kim HJ, Bak KH, Chun HJ, Oh SJ, Kang TH, Yang MS. Posterior interspinous fusion device for one-level fusion in degenerative lumbar spine disease: comparison with pedicle screw fixation—preliminary report of at least one year follow up. J Korean Neurosurg Soc. 2012;52(4):359–64. https://doi.org/10.3340/jkns.2012.52.4.359.
Postacchini F, Postacchini R, Menchetti PPM, Sessa P, Paolino M, Cinotti G. Lumbar interspinous process fixation and fusion with stand-alone interlaminar lumbar instrumented fusion implant in patients with degenerative spondylolisthesis undergoing decompression for spinal stenosis. Asian Spine J. 2016;10(1):27–37. https://doi.org/10.4184/asj.2016.10.1.27.
Falowski SM, Mangal V, Pope J, et al. Multicenter retrospective review of safety and efficacy of a novel minimally invasive lumbar interspinous fusion device. Pain Res. 2021;31(14):1525–31. https://doi.org/10.2147/JPR.S304957.
Fairbank JCT, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000;25(22):2940–53.
Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94.
Buckley DI, Eckstrom E, Morris C, et al. Performance of a patient reported outcomes measurement information system (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med. 2015. https://doi.org/10.1093/pm/pnv046.
Amtmann D, Kim J, Chung H, Askew R, Park R, Cook K. Minimally important differences for PROMIS pain interference for individuals with back pain. J Pain Res. 2016;9:251–5.
Chen CX, Kroenke K, Stump TE, et al. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. 2018;159(4):775–82.
Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 patient-reported outcomes measurement information system short forms: physical function, pain interference, depression, and anxiety in knee osteoarthritis. J Pain. 2017;18(9):1096–110.
Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–5.
Schmidt S, Franke J, Rauschmann M, Adelt D, Bonsanto M, Sola S. Prospective, randomized, multicenter study with 2-year follow-up to compare the performance of decompression with and without interlaminar stabilization. J Neurosurg Spine. 2018;28(4):406–15.
Sclafani JA, Liang K, Ohnmeiss DD, Gordon C. Clinical outcomes of a polyaxial interspinous fusion system. Int J Spine Surg. 2014;8:35.
Condez B, Parrish R, Camisa W, Leasure JM, Randall JC. Stability and decompression mechanics of several MIS lumbar fixation technologies: a biomechanical study. 2015. https://www.semanticscholar.org/paper/Stability-and-Decompression-Mechanics-of-Several-A-Condez-Parrish/28db8c3dfde9e48f83add9eeed9de36be38ef7f0. Corpus ID: 209753176.
Pope JE, Fishman M, Chakravarthy K, et al. A retrospective, multicenter, quantitative analysis of patients’ baseline pain quality (PROMIS 29) entering into pain and spine practices in the United States: the ALIGN study. Pain Ther. 2021;10:539–50.
Acknowledgements
We thank the participants of the study.
Funding
This study was sponsored by Aurora Spine. Aurora Spine also funded the Rapid Service Fee. No other funding source was present. Aurora Spine had no access to the data or for the writing of this manuscript.
Editorial and Other Assistance
Eric Bruntlett, Pacific Spine Research Institute (PRI), Shelley Trimm (PRI), Kamren Murrell (PRI) provided project management and statistical analysis; Sarah Martineck (Aurora Spine) provided manuscript creation and editing support. All support was funded by Aurora Spine.
Author Contributions
Drs. Steven M. Falowski and Jason E. Pope were responsible for concept and design, primary manuscript preparation, review and edits. Dr. Vip Mangal participated in concept/design, manuscript preparations, review and edits. Drs. Louis J. Raso, Ali Narizi, Denis G. Patterson, Yousseff Josephson, Michael D. Danko, Sebastian Koga, Rafael Justiz and Rainer S. Vogel participated in manuscript preparation, review and edits of the manuscript.
Disclosures
Dr. Steven M. Falowski serves as a consultant for Abbott, Medtronic, Saluda, VertiFlex, Vertos, Surgentec, CornerLoc, Mainstay and Relievant, has received grant for research funding from Mainstay, Relievant, Medtronic, Abbott, VertiFlex, Saluda, Nalu, CornerLoc, Aurora, Biotronik, and Stimgenics, and has an equity position in SynerFuse, Aurora Spine, Thermaquil. SPR Therapeutics, Saluda, CornerLoc, PainTEQ, Stimgenics, Anesthetic Gas Reclamation, Neural Integrative Solutions, SpineThera, and Celeri Health. Dr. Louis J. Raso serves on the speakers Bureau for Boston Scientific, Aurora Spine, SurGen Tec, Vertiflex, and FloSpine. He is a Stockholder for Aurora Spine and receives Royalties from Flospine and SurGen Tec. Dr.Vipul Mangal serves as a consultant for Medtronic, Aurora and has received educational support from Medtronic, Abbott, Boston Scientific, Nevro, Vertos, Stimwave, Nalu, Aurora, Spinal Simplicity, SI Fix. Dr. Ali Narizi serves as a Consultant for Nevro, Flowonix, Boston Scientific, Surgentec, Aurora Pain and Spine; has received research support from Nevro, Corner Loc, Cardio Surgical Partners, Flowonix. Dr. Denis G. Patterson serves as a consultant for Abbott, AIS, Allergan, Amgen, Aurora Spine, CornerLoc, Flowonix, Lundbeck, Pajunk Medical, Saluda, Spark Biomedical, Vertos, has received grant and research support from Abbott, Flowonix, Nevro, Saluda and Vertiflex, serve on the Speaker Bureau or Honoraria for Abbott, Allergan, Amgen, CornerLoc, Lundbeck, Saluda, Vertos, and has equity in CornerLoc. Dr. Michael D. Danko is a consultant for Medtronic, Abbott, and Aurora spine and has Stock Options at Aurora Spine. Dr. Rafael Justiz serves as a consultant for Abbott, Medtronic, and has research or grant support from Medtronic and Stimgenics. Dr. Rainer S. Vogel serves as a consultant for Medtronic, Nevro, BSCI and Aurora Spine; teaching agreements with Medtronic, Nevro, BSCI, and Aurora Spine; research associated funding with Medtronic, Nevro, BSCI, PainTEQ, Aurora Spine, Vertos, and Vertiflex; Investments with Medtronic, Nevro and Aurora Spine. Dr. Sebastian Koga is a consultant for Aurora Spine Corp., Synpative Medical Inc., and Osseus Spine; and a shareholder in Aurora Spine Corp. and Synpative Medical Inc. Dr. Youssef Josephson serves as a consultant for Medtronic, Aurora, Abbott, NALU, Sprint, and PainTEQ; research associated funding with Aurora, Abbott, Sprint, and PainTEQ. Dr. Jason E. Pope serves as a consultant for Abbott, Medtronic, Saluda, Flowonix, SpineThera, Painteq, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, Thermaquil; has received grant and research support from: Abbott, Flowonix, Saluda, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, Thermaquil; and is a shareholder of: PainTEQ, Vertos, SPR Therapeutics, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil and Anesthetic Gas Reclamation.
Compliance with Ethic Guidelines
IRB approval was provided by Western IRB (IRB #20211168). All patient data collected were de-identified to provide patient data confidentiality and compliance with the Declaration of Helsinki. The protocol and IRB were each approved by the local governing entities of each involved institution. All enrolled subjects provided voluntary written informed consent to participate. Subjects were allowed to ask questions and were given a copy of the informed consent.
Data Availability
No public access to data sets are available as the study is still in process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Falowski, S.M., Raso, L.J., Mangal, V. et al. A Prospective, Observational, Open-Label, Non-Randomized, Multicenter Study Measuring Functional Outcomes in a Novel Interspinous Fusion Device in Subjects with Low Back Pain: REFINE Study. Pain Ther 12, 187–199 (2023). https://doi.org/10.1007/s40122-022-00447-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00447-0