Abstract
Introduction
In this single-center retrospective cohort study, we investigated the efficacy of letermovir in preventing Cytomegalovirus (CMV) infection in patients with aplastic anemia (AA) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods
Based on whether or not letermovir was used for preventing CMV infection, the patients were categorized into two groups: letermovir and control groups. The overall survival (OS) rate and cumulative incidence of CMV infection during the first 100 days after allo-HSCT were evaluated. The study included 21 matched pairs of patients, identified through propensity score matching analysis, to compare CMV infection rates, treatment efficacy, and regression.
Results
The incidence of CMV infection within 100 days after transplantation was significantly lower in the letermovir group than in the control group (26.5 vs. 77.4%, respectively; P < 0.001), among a total of 87 patients who underwent the transplant. In the matched cohort of 21 patients with AA, the letermovir group also showed a significantly reduced cumulative incidence of CMV infection (14.3 vs. 90.5% in the control group; P < 0.001). Compared to the control group, patients with CMV infection in the letermovir group had lower CMV-DNA load and a shorter clearance time. However, there was no significant difference in OS between both groups (P = 0.34).
Conclusions
Letermovir effectively prevents CMV infection in allo-HSCT recipients with AA and demonstrates a high safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Aplastic anemia (AA) patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) are at high risk for Cytomegalovirus (CMV) infection, which can lead to severe complications and affect the overall success of the transplantation. |
Effective prevention strategies for CMV infection post-transplantation are crucial to improve patient outcomes, but the specific efficacy and safety of letermovir in patients with AA have not been extensively studied. |
What was learned from the study? |
The study demonstrated that letermovir significantly reduced the incidence of CMV infection within the first 100 days after allo-HSCT in patients with AA. |
Letermovir showed a high safety profile, with most patients tolerating the treatment well and experiencing a reduction in CMV-DNA load and infection clearance time. |
Introduction
Aplastic anemia (AA) is a severe hematopoietic disorder characterized by rapid onset and progression. Currently, the principal intervention for AA is allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1]. Cytomegalovirus (CMV) is among the most common opportunistic infections that occur in patients post-HSCT. Early CMV reactivation can occur in 70% of patients post-HSCT during the immunocompromised phase [2, 3]. Significantly, CMV reactivation is associated with increased non-relapse mortality [4]. Green et al. showed that CMV-DNA load > 500 IU/ml increased the risk of death by 20-fold within 60 days of transplantation [3].
The rapid advances in HSCT techniques in recent decades have broadened the indication of HSCT in AA recipients. The donor spectrum has evolved from human leukocyte antigen (HLA)-matched sibling donors (MSD) to include HLA-haploidentical donors (HID) and HLA-matched unrelated donors (MUD) [5]. The transplant conditioning regimen usually contains antithymocyte globulin (ATG) for AA, which is unique to nonmalignant hematological diseases. In addition, immunosuppressive therapy with cyclosporine A is generally continued for more than 1 year following transplantation to prevent graft rejection and graft-versus-host disease (GVHD), which leads to delayed immune activation and increases the susceptibility to infection by various pathogens [6, 7]. Compared to other hematological malignancies, patients with AA undergoing HSCT demonstrate a higher incidence of CMV infection. The incidences of CMV infection after AA MSD-HSCT, unrelated donor HSCT (MUD-HSCT), and haploidentical-HSCT (HID-HSCT) were 43.5–55.3%, 66.7–82.6%, and 51.7–80%, respectively [5, 8,9,10]. Preventing CMV reactivation is crucial for improving patient prognosis after allo-HSCT, given its high morbidity and associated mortality, with most CMV infections occurring within the first 100 days following allo-HSCT [11]. Therefore, formulating effective CMV prevention and treatment strategies is imperative. Currently, although some drugs are effective in preventing and treating CMV infection in patients with AA, they have serious adverse effects, such as myelosuppression, nephrotoxicity, and drug resistance, which limits their clinical application.
On November 8, 2017, the US Food and Drug Administration approved letermovir for preventing and treating CMV infection and for the treatment of CMV-seropositive individuals in adults undergoing allo-HSCT [12, 13]. In phase III clinical trials, the rate of CMV infection and the incidences of all-cause mortality in patients who underwent transplantation treated with letermovir as a prophylactic agent were low compared to the placebo group, with a high safety profile [12]. However, most studies on letermovir have not differentiated between patients with hematological malignancies and those with AA, who differ significantly in treatment regimens and transplantation-related complications. Currently, there are still limited data on the use of letermovir for preventing CMV infection after allo-HSCT in patients with AA. Therefore, in this single-center retrospective cohort study, we investigated the efficacy of letermovir in preventing CMV infection among patients with AA who have undergone allo-HSCT and compared its effects with control drugs.
Methods
Patients
Patients with AA undergoing allo-HSCT at Guangzhou First People’s Hospital from January to December 2022 were included. The inclusion criterion was patients who tested positive for CMV antibodies before HSCT (R +). Follow-up data were collected from outpatient/inpatient medical records and telephone interviews until April 30, 2023. The exclusion criteria were as follows: (1) patients who died within 30 days post-HSCT and (2) patients who had active CMV DNAemia at the time of letermovir initiation. In this study, CMV-seropositive patients with a CMV viral load of < 500 copies/ml were not classified as having a CMV infection. The use of letermovir, informed by discussions on its costs, potential side effects, and research findings, was decided by patients and their families, adhering to our practice of patient autonomy and informed consent. This study was approved by the Ethics Committee of Guangzhou First People’s Hospital and conducted in accordance with the principles of the Declaration of Helsinki.
Transplant-Related Treatments
Appropriate conditioning regimens were selected based on factors such as the patient’s disease course and donor source. The posttransplant cyclophosphamide (PTCy) regimen included cyclophosphamide (Cy), ATG, fludarabine (Flu), with or without total body irradiation (TBI). The PTCy + Bu regimen involved Cy, ATG, Flu, and busulfan (BU). PTCy and PTCy + Bu regimens were augmented with post-transplant cyclophosphamide. For the BuCy/FCA regimens, either BU plus CY plus ATG, or Flu plus CY plus ATG were used. All patients were treated with glucocorticoids to prevent possible seropathy from ATG therapy. In addition, the patients were treated with short-course methotrexate, cyclosporine, or tacrolimus to prevent GVHD. Patients undergoing MUD-HSCT and HID-HSCT were further supplemented with mycophenolate mofetil. The combination of donor bone marrow and peripheral blood stem cells (PBSCs) was used as grafts for patients who underwent MSD-HSCT and HID-HSCT, and donor PBSC was used for those who underwent MUD-HSCT.
CMV Monitoring
We monitored for CMV infection after successful neutrophil transplantation based on the CMV-DNA load, which was determined using a quantitative polymerase chain reaction on plasma samples. The CMV-DNA load was monitored 2–3 times per week at regular intervals for 3 months, and then weekly for 6 months after allo-HSCT. Then, the number and interval for testing the CMV-DNA load were determined based on the physician’s judgment. The lower limit of CMV-DNA load was 500 copies/ml. Furthermore, patients with CMV infection underwent routine chest computed tomography scans, pulmonary function tests, and fundus examinations. CD16 + CD56 + natural killing (NK) cells, T cell subsets (CD3 + , CD4 + , CD8 +), CD19 + B cells, and serum immunoglobulin concentrations (IgM, IgG, IgA) were analyzed using flow cytometry and scatter turbidimetry at first, second, and third month after allo-HSCT.
Definitions of CMV Infection and CMV Disease
CMV infection was defined as any evidence of CMV activation and CMV disease. Herein, CMV-DNA load > 500 copies/ml on two consecutive tests suggested CMV activation. CMV disease was defined as the presence of CMV infection with concurrent CMV-related impairment of organ function, including end-organ involvement, which typically manifests as enteritis, pneumonia, retinitis, hepatitis, and encephalitis according to the published recommendations [14, 15].
Prevention and Treatment of CMV
For CMV prophylaxis, patients in the letermovir group were administered 240 mg/day of letermovir tablets from the day of neutrophil engraftment post-HSCT, with the dosage adjusted to 240 mg every other day for children. This regimen continued until day + 100 post-transplantation, aiming to offer optimal protection against CMV infection during the early post-transplant period. The control group only received conventional preemptive treatment after allo-HSCT. If CMV infection is detected in a patient, prophylactic medication is discontinued, and intravenous antiviral treatment is initiated. Ganciclovir and foscarnet are administered intravenously as appropriate, with ganciclovir being the preferred drug. If a patient cannot tolerate ganciclovir due to low blood cell counts, or if ganciclovir proves ineffective, foscarnet is used instead. Ganciclovir was administered at a dose of 5 mg/kg body weight twice daily intravenously, and foscarnet was administered at a dose of 80 mg/kg body weight thrice daily. Simultaneously, CMV-DNA load was measured 2–3 times weekly. If the results of two continuous tests showed a CMV-DNA load of < 500 copies/ml, antiviral therapy was discontinued. In addition, patients were treated based on the infection site; for instance, patients with CMV retinitis were treated with intravenous and vitreous cavity injections of ganciclovir. CMV clearance was defined as undetectable CMV-DNA in the blood or CMV-DNA load below the lower limit of quantification (500 copies/ml) in two consecutive tests performed at an interval of several days.
Statistical Analyses
All statistical analyses were performed using SPSS software (version 23.0, IBM Corp., Armonk, NY) and R software (version 3.6.1, https://www.r-project.org/). Propensity score matching (PSM) analysis was executed using the MatchIt and Table 1 packages. The Mann–Whiney test and χ2 test were respectively used for continuous and categorical variables to compare differences between groups. The last follow-up date for all surviving patients was April 30, 2023. The overall survival (OS) analyses were performed using the Kaplan–Meier method and log-rank tests. The primary endpoint was the number of patients with clinically significant CMV infection. The cumulative incidence of CMV infection was evaluated using Gray’s test. Univariate and multivariate COX regression analyses were performed to determine the risk factors of CMV infection. All factors with a significance of P < 0.1 in the univariate analysis were evaluated in the multivariate analysis. Factors were considered independently predictive of outcomes when a significant association was observed, and P < 0.05 was considered statistically significant.
Results
Patient Characteristics and Survival
A total of 87 patients (44 male, 50.6%; 43 female, 49.4%) were included. All 87 patients were CMV-seropositive, with 82 of their donors being CMV-seropositive and five CMV-seronegative. Of the total, 31 patients underwent MSD-HSCT, 19 underwent MUD-HSCT, and 37 underwent HID-HSCT. A total of 53 patients in the control group did not receive letermovir for CMV infection prevention, as compared to 34 patients in the letermovir group. The information on a specific patient is shown in Table 1. To reduce the effect of potential confounding factors, PSM analysis was performed for patients treated with either letermovir or acyclovir tablets for preventing CMV infection. The propensity score was calculated based on the patient’s sex and age at transplantation, diagnosis, pre-transplantation Eastern Cooperative Oncology Group score, donor’s age and sex, treatment regimen prior to transplantation, donor source, and compatibility of donor-recipient blood groups. A total of 21 matched pairs of cases were included, wherein all variables were balanced in both groups after matching (P > 0.05). The basic characteristics of the patients with AA before and after matching are shown in Table 2. During the observation period, among the 34 patients who received letermovir for CMV prophylaxis, only one patient discontinued the medication due to significant gastrointestinal side effects, particularly diarrhea. The symptoms subsided after discontinuation of letermovir, and no other serious adverse reactions were observed. Among the 87 patients, two patients died during the follow-up period due to severe pneumonia and sepsis. The median follow-up duration was 267 days (range, 100–478 days). The OS rate was 97.7%, with no significant difference in OS between the groups (P = 0.34; Fig. 1).
CMV Infection and Risk Factors
Within the first 100 days after allo-HSCT, among the 34 patients who received letermovir, nine (26.5%) developed CMV infection, while 25 (73.5%) remained infection-free. In contrast, 41 (77.4%) of the 53 patients in the control group developed CMV infection. The cumulative incidence of CMV infection within 100 days after transplantation was significantly different between both groups (P < 0.0001; Fig. 2), and the median duration for developing CMV DNAemia was 38.5 days (range, 7–98 days) post-transplant. In this study, multiple factors such as sex and disease duration were analyzed using Cox regression analysis to identify the risk factors for CMV infection within 100 days after allo-HSCT. The factors with P values < 0.1 in the Cox regression univariate analysis were included in the multifactor analysis. According to the analysis, letermovir and younger age at HSCT (< 30 years) were statistically significant protective factors against CMV infection occurring within 100 days after transplantation (Table 1). In the matched group analysis (42 patients), the letermovir group showed a significantly lower incidence of CMV infection (14.3 vs. 90.5% in the control group, P < 0.001; Fig. 3). No cases of CMV disease affecting organ function were observed in the letermovir group. The median maximum peak value of CMV-DNA load was 1900 copies/ml (1070–2270 copies/ml) for letermovir patients and 3060 copies/ml (808–9910 copies/ml) for the control group (P = 0.0165; Fig. 4), indicating a higher viral load in the control group. Additionally, two control-group patients developed CMV retinitis, evidencing the severity of CMV infection in this group.
Regression of CMV Infection
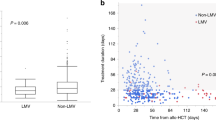
The mean duration from diagnosis to clearance of CMV infection in the letermovir and control groups was 11.7 and 18.6 days, respectively, which was significantly different (P = 0.0219; Fig. 5). Although three patients in the letermovir group were diagnosed with CMV infection, none experienced organ function impairment or involvement until the end of the follow-up. Conversely, two of 18 patients in the control group with CMV DNAemia developed CMV retinitis.
Immune Function Reconstitution within 100 days after allo-HSCT
One month after HSCT, no statistical differences were observed in the absolute number of T cell subsets (CD3+, CD4+, CD8+), CD19+B cells, CD16+CD56+NK cells, and serum immunoglobulin concentrations (IgM, IgG, IgA) levels between the letermovir and control groups. Two months after transplantation, there were higher CD3+ and CD8+ T lymphocytes in the control group, with statistical differences between both groups. Three months after transplantation, there were statistical differences in CD19+ B cells, CD16+CD56+ NK cells, and IgG in both groups, which were higher in the control group. Overall, patients in the control group had higher reconstitution of immune function after transplantation than in the letermovir group Fig. 6.
Discussion
CMV belongs to the family of human herpesviruses (HHVs), and it is also known as HHV-5. CMV remains latent in approximately 40–70% of children and 60–90% of adults [15]. CMV activation by the recipient or donor source is observed in patients after allo-HSCT, which causes direct and indirect virulence and increases the risk of bacterial and fungal infections, thereby suppressing the immune system [16].
AA presents a severe and acute condition where allo-HSCT stands as the primary treatment. Prior to transplantation, patients with AA are immunocompromised due to disease characteristics, which increases their risk of developing various potential infections. Typically, a nonmyeloablative conditioning regimen involving immunosuppressive agents is employed in AA transplantation. A prolonged post-transplantation administration of immunosuppressive drugs is compulsory to prevent rejection and treat GVHD, leading to a slower re-establishment of immune function and a relatively higher rate of CMV infection than in patients with hematologic malignancies after transplantation [6, 7]. In our center, the incidence of CMV infection in patients with AA was 84.8% after 100 days of transplantation before using letermovir as a prophylactic agent. Furthermore, CMV infections are more prone in substitute donor transplants than MSD-HSCT, and the median time onset of CMV infection is 30 days after allo-HSCT. Consequently, our center initiated CMV prophylaxis with letermovir from the day of neutrophil engraftment following transplantation. As CMV activation and CMV disease-related morbidity were found to be associated with transplantation-related mortality, preventing, detecting, and treating CMV infection is crucial, especially during the early stage after transplantation [3, 17]. This will help reduce the indirect effects of potential CMV, such as the increased risk of other opportunistic infections, disease-free progression, and reduced mortality, thereby improving the success of transplantation [2].
The choice of a treatment strategy to prevent CMV infection primarily depends on factors such as the status of CMV-DNA load in serum, other transplantation-related risks, and the cost of antiviral drugs. Some antiviral drugs have serious adverse effects, such as myelosuppression, nephrotoxicity, and drug resistance, limiting their clinical application. With the advancement and increased use of substitute donor transplant techniques in recent years, there is an urgent need to design effective strategies to prevent CMV infection. Regular monitoring after transplantation facilitates early detection of viral replication. Antiviral therapy should be initiated when viral load values exceed predetermined threshold values to avoid symptomatic infections. Our transplant center uses a mixed approach to reduce drug costs. Patients in the letermovir group were given aggressive prophylactic agents in the early post-transplant phase when the risk of infection was the highest. The decision to continue using letermovir was based on the patient’s discretion and economic status. Patients who discontinued letermovir were given oral acyclovir tablets for prophylaxis.
Letermovir inhibits the CMV DNA terminase complex, which is required for viral DNA processing and packaging, and disrupts the assembly of viral genes. It has no side effects, such as myelosuppression and nephrotoxicity, which are commonly observed with other antivirals. This is particularly beneficial for patients with AA, who often experience slow hematologic recovery and immune reconstitution. Based on the latest foreign guidelines and clinical experience, in 2020, the best approach to prevent CMV infection after allo-HSCT was to use letermovir as a specific antiviral prophylactic agent. Currently, letermovir is approved worldwide for preventing CMV seropositivity in adults after allo-HSCT [6, 12]. Furthermore, studies have shown that letermovir has a good safety and efficacy profile in pediatric and adolescent patients who underwent transplantation [18,19,20,21]. In our study, 1 of 5 children or adolescents aged < 18 years in the letermovir group developed CMV infection without progression to CMV disease. No adverse drug reactions were observed, and the tolerability and safety profile of letermovir were comparable to other studies and clinical trials [20, 21]. In our study, three patients in the letermovir group developed CMV infection with peak CMV-DNA values of 1900 copies/ml, 1070 copies/ml, and 2270 copies/ml, respectively, without progression to CMV disease. The overall viral load of patients in the letermovir group was lower after CMV infection as compared to the control group, and the time required for treatment was shorter. Thirty-four patients were given letermovir to prevent CMV infection until the end of follow-up. Only one patient discontinued the drug due to a significant gastrointestinal reaction (diarrhea); however, the symptom was resolved after the drug was discontinued. Letermovir demonstrated a good tolerability and safety profile in clinical settings, in line with other studies [12, 22,23,24]. Furthermore, previous studies have reported a correlation between the incidence of CMV infection and immune function reconstruction following transplantation, with patients having low immune function being more susceptible to CMV infection [25]. Patients with AA are immunocompromised and have slow immune function recovery due to the disease characteristics and often require immunosuppressive drugs during and after transplantation. These patients have a higher incidence of CMV infection as compared to those with other hematologic malignant diseases. In this study, patients in the letermovir group have lower immune function than the control group within 100 days after allo-HSCT. This may be because doctors are increasingly advocating for the use of letermovir in immunocompromised patients who are at a high risk of developing infections in clinical practice. The incidence of CMV infection was significantly lower in the former group, indicating a preventive effect of letermovir in immunocompromised patients. There are some studies, however, that have shown that prophylaxis with letermovir is associated with a reduction in CMV-specific immune reconstitution after transplantation [26]. However, the overall mortality rate of patients in both groups was not significantly different, which may be attributed to the small sample size and short follow-up duration. Using letermovir as a primary prophylaxis in patients with AA after allo-HSCT significantly reduces CMV infection and demonstrates a high safety profile. Therefore, letermovir is currently used as a prophylactic for patients with AA undergoing allo-HSCT in our institution.
At present, primary prophylaxis with letermovir within 100 days after transplantation is the standard protocol for CMV-seropositive allo-HSCT recipients, as endorsed by the latest CMV prophylaxis guidelines of The American Society of Transplant and Cellular Therapy [27]. Letermovir discontinuation significantly increased the cumulative incidences of CMV infection within 200 days post-transplantation. As this study utilized letermovir for a short period, CMV infection occurring only within 100 days after transplantation was analyzed. The incidence of CMV infection in patients beyond the 100-day timeframe is still being monitored. Therefore, the timing of drug discontinuation should be further explored. Ongoing randomized clinical trials have extended the duration of prophylaxis in high-risk patients to 200 days post-transplantation, wherein the effects and benefits should be further explored. In this study, we have not explored the efficacy of letermovir therapy for treating pre-existing CMV infection and resistance to letermovir. A study has shown a low success rate for treating patients with pre-existing CMV activation using letermovir [23]. Studies investigating the use of letermovir for treating CMV infection after transplantation are limited. Here, we treated a few patients with letermovir, but they were excluded from this study due to the small sample size and short follow-up duration.
Although the patients’ overall tolerability to letermovir was good, the possibility of other adverse events exists due to the complex drug interactions [6]. In our center, patients were not prevented from letermovir for a long time, and the follow-up duration was short. Therefore, the long-term effects and cumulative incidences of CMV infection must be monitored continuously. In addition, the viral load of patients in the letermovir group at the time of CMV infection was lower compared to the control group. The treatment duration of patients in the letermovir group with intravenous antiviral drugs was short. Treating CMV infection poses a significant economic burden on patients. Hence, further pharmacoeconomic analyses comparing preventive strategies in transplant populations are essential to determine the role of letermovir in CMV management [24].
Our study has one limitation, which is its small sample size. Therefore, future large sample-sized studies exploring the effect of letermovir in preventing CMV infection after HSCT are warranted.
Conclusions
CMV infection is a dangerous complication in patients with AA undergoing allo-HSCT. Researchers have attempted to balance the risk of CMV infection and the toxicity of therapeutic agents, considering the adverse effects and resistance to previous nucleoside analogs. Letermovir offers a new option for managing CMV infection in patients post-HSCT due to its unique mechanism of action. In addition, it has demonstrated a high safety profile and effectiveness in preventing and treating CMV infection in patients with AA post-transplantation, especially in patients with slow reestablishment of immune function.
Availability of Data and Materials
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70.
Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–58.
Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–27.
Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–38.
Xu LP, Jin S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10(1):25.
Saullo JL, Miller RA. Cytomegalovirus therapy: role of letermovir in prophylaxis and treatment in transplant recipients. Annu Rev Med. 2023;74:89–105.
Mo W, Chen X, Zhang X, et al. The potential association of delayed t lymphocyte reconstitution within six months post-transplantation with the risk of cytomegalovirus retinitis in severe aplastic anemia recipients. Front Cell Infect Microbiol. 2022;12:900154.
Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–24.
Zhang Y, Wu L, Mo W, et al. Comparable outcomes of first-line hematopoietic stem cell transplantation from unrelated and matched sibling donors in adult patients with aplastic anemia: a retrospective single-center study. Biol Blood Marrow Transplant. 2019;25(8):1567–75.
Zhang YY, Mo WJ, Zuo YY, et al. Comparable survival outcome between transplantation from haploidentical donor and matched related donor or unrelated donor for severe aplastic anemia patients aged 40 years and older: A retrospective multicenter cohort study. Clin Transplant. 2020;34(3): e13810.
Blyth E, Withers B, Clancy L, et al. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967–80.
Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–44.
Verghese PS, Schleiss MR. Letermovir treatment of human cytomegalovirus infection antiinfective agent. Drugs Future. 2013;38(5):291–8.
Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–7.
Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91.
Powers C, Defilippis V, Malouli D, et al. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–59.
Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59(4):473–81.
Styczyński J, Czyżewski K, Dębski R. Primary prophylaxis with letermovir for prevention of CMV infection in two children. Acta Haematol Pol. 2020;51(4):263–4.
Chiereghin A, Belotti T, Borgatti EC, et al. Off-label use of letermovir as preemptive anti-cytomegalovirus therapy in a pediatric allogeneic peripheral blood stem cell transplant. Infect Drug Resist. 2021;14:1185–90.
Richert-Przygonska M, Jaremek K, Debski R, et al. Letermovir prophylaxis for cytomegalovirus infection in children after hematopoietic cell transplantation. Anticancer Res. 2022;42(7):3607–12.
Daukshus NP, Cirincione A, Siver M, et al. Letermovir for cytomegalovirus prevention in adolescent patients following hematopoietic cell transplantation. J Pediatric Infect Dis Soc. 2022;11(7):337–40.
Mori Y, Jinnouchi F, Takenaka K, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transplant. 2021;56(4):853–62.
Anderson A, Raja M, Vazquez N, et al. Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transplant. 2020;34(7): e13866.
Derigs P, Radujkovic A, Schubert ML, et al. Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol. 2021;100(8):2087–93.
Wang LL, Mo WJ, Zhang YP, et al. Clinical analysis of cmv infection after allogeneic hematopoietic stem cell transplantation in severe aplastic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(3):944–50.
Zamora D, Duke ER, Xie H, et al. Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood. 2021;138(1):34–43.
Hakki M, Aitken SL, Danziger-Isakov L, et al. American society for transplantation and cellular therapy series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(9):707–19.
Funding
This study was supported by grants from the Innovative Clinical Technique of Guangzhou (No. 2019GX04) (YPZ), 2019 Annual Research Project of The China Marrow Donor Program (No. CMDP201902) (SQW), Guang Zhou Basic and Applied Basic Research Foundation (202201010062) (CXW), Guangzhou Municipal Science and Technology project (202002030035) (SQW), Natural Science Foundation of Guangdong Province (2018A030130179) (MZ) and Science and Technology Projects in Guangzhou (202201010062) (XWC). The journal’s rapid service fee was funded by Science and Technology Projects in Guangzhou (202201010062) (XWC).
Author information
Authors and Affiliations
Contributions
WJM, and SQW: Contributed to the concept development and study design; coordinated the research and helped to write the manuscript. YLZ and XWC: Collected the clinical information, analyzed the data, and wrote the manuscript. MZ, YPZ, CTC, RQZ, YML, FFY, SLX, CXW, WZ, TFD, and SYP: Diagnosed and treated the patients, provided clinical information, and contributed to the follow-up of patients. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Guangzhou First People’s Hospital and conducted in accordance with the principles of the Declaration of Helsinki.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Y., Chen, X., Zhou, M. et al. Letermovir Effectively Prevents Cytomegalovirus Infection in Patients with Aplastic Anemia After Hematopoietic Stem Cell Transplantation: A Real-World Retrospective Cohort Study. Infect Dis Ther 13, 345–359 (2024). https://doi.org/10.1007/s40121-024-00917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00917-2