Abstract
Introduction
Chronic hepatitis B (CHB) is one of the world's major healthcare problems, especially in the Western Pacific regions. This study describes the prevalence, incidence, treatment profiles and clinical and economic burden of chronic hepatitis B patients in Japan using the Japan Medical Data Center (JMDC) Claims Database.
Methods
This is a retrospective observational study. Prevalence cases were identified as patients with ≥ 1 inpatient or ≥ 2 outpatient CHB diagnoses and ≥ 2 records for hepatitis B tests or ≥ 1 prescription for CHB treatment between January 2010 and December 2019. Newly diagnosed CHB patients were defined as patients diagnosed from 2010 to 2018 with no history of the disease up to 2 years prior to the diagnosis. The index date is defined as the first CHB diagnosis day. We only used patients’ data with ≥ 1-year post-index date.
Results
We identified 13,061 CHB prevalent cases (2010–2019), yielding a crude period prevalence of 0.32%. Newly diagnosed CHB patients (n = 1973; median age 52 years) were followed for a median period of 3.1 years, during which 15% received a CHB treatment. Entecavir was the most common first treatment (66%). During this period, 3.4% of the patients developed compensated cirrhosis (CC), 1.5% decompensated cirrhosis (DC) and 3.0% hepatocellular carcinoma (HCC). Around 43.3% of CHB patients were hospitalized at least once. Hospitalizations, treatment rates, serologic testing and screening for liver diseases increased as the severity of the disease progressed. The average total healthcare cost was 870,568 JPY (7779 USD) per person per year. DC and HCC resulted in the highest management costs.
Conclusions
Chronic hepatitis B represents a high clinical and economic burden for patients and caregivers, given its morbidity and associated costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study assessed the prevalence, incidence, treatment profiles and clinical and economic burden of chronic hepatitis B (CHB) patients in Japan using the JMDC Claims Database. |
The prevalence of CHB patients in this study covering the period between 2010 and 2019 was 0.32%, and mean age was 51.1 years. |
The average total healthcare cost was 870,568 JPY (7779 USD) per person per year. Decompensated cirrhosis (DC) and hepatocellular carcinoma (HCC) resulted in the highest management costs. |
Introduction
The World Health Organization estimates that in 2015, 257 million persons (3.5% of the population) were living with chronic hepatitis B infection (CHB) worldwide. The prevalence was the highest in Western Pacific regions (especially Taiwan, Japan, South Korea and China) where 6.2% of the adult population is chronically infected [1]. In Japan, the prevalence of CHB in 2016 was estimated to be around 0.6% based on pooled data from all eligible studies reporting prevalence of hepatitis B virus antigen (HBsAg) in the general population [2].
CHB can have a direct bearing on life expectancy due to progression to liver cirrhosis, liver failure and/or hepatocellular carcinoma (HCC). Apart from the liver-related sequelae of CHB, non-liver comorbidities are prevalent in CHB patients with the most common being cardiovascular diseases and diabetes [3].
The main goal of CHB therapy is to prevent disease progression. Currently pegylated interferon (Peg-IFN) and nucleos(t)ide analogs (NAs) are employed in antiviral therapy for CHB. PegIFN-α-2a and NAs such as entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) are approved in Japan and have been recommended for treatment. Despite the availability of vaccines and treatment options, there is considerable unmet need in the real world, potentially driven by poor or delayed diagnosis, suboptimal adherence to often lifelong treatment and low rates of sustained HBsAg loss (or ‘functional cure,’ which further reduces the risk of progression to HCC). Real-world data on how CHB patients are managed in Japan following the marketing of new drugs and the update of CHB clinical practice guidelines are also needed [4].
In addition to the clinical burden of the disease, CHB is associated with significant economic burden in Asian countries with direct cost being correlated with disease severity [3, 5,6,7,8]. Medical resources such as non-antiviral medications, laboratory tests and imaging examinations, especially in patients with cirrhosis and HCC, account for a significant fraction of direct medical costs [8].
This study aims to provide real-world evidence about CHB epidemiology, disease burden, treatment patterns and real-life data on healthcare resource utilization (HCRU) and costs by key stages of disease progression in Japan using data for newly diagnosed CHB patients derived from the Japan Medical Data Center (JMDC) Claims database.
Methods
Data Source
The study utilized 2008–2020 de-identified data from the JMDC Inc. (Japan) Claims database. Based on Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labour and Welfare, ethics approval was not applicable to this study. This database included approximately 7.3 million (as of April 2020) insured persons (approximately 5.8% of the population) from multiple health insurance associations, comprised mainly of company employees and their family aged between 20 and 74 years [9, 10]. The database includes monthly claims for health insurance reimbursement from medical institutions and pharmacies since 2005 and provides information on the beneficiaries [encrypted personal identifiers, age, sex, International Classification of Diseases 10th revision (ICD-10) procedure and diagnostic codes, and details of prescription] [9, 10].
Study Design and Population
This is a retrospective observational study on patients diagnosed with CHB derived from the JMDC Claims database. The primary outcomes of this study were the estimation of the prevalence of CHB and healthcare resource utilizations and costs and disease progression (health state) of the newly diagnosed CHB patients. The secondary outcome was the description of the newly diagnosed CHB patients in terms of demographic and clinical characteristics, and treatment patterns.
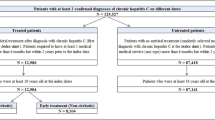
CHB cases were identified based on a case selection algorithm developed with the hepatology expert in this study: patients with at least one inpatient or two outpatient claim for CHB diagnosis [ICD-10 code B18.0 or B18.1) between January 2010 and June 2020 and presence of either two records for HBV-specific tests [HBsAg, HBV DNA or hepatitis B virus e antigen (HBeAg)] or prescription for CHB treatment during this period. Cases diagnosed with hepatitis C virus or acute hepatitis B during this period were excluded. Among the remaining patients, those with CHB diagnosis falling between 2010 and 2018 (to allow a minimum of follow-up) with no history of the disease (inpatient or outpatient diagnosis code of CHB) up to 2 years prior the diagnosis (from 2008 to year N) formed our main study population (incident cohort) (Fig. 1).
CHB incident patients were evaluated longitudinally from the index date (the first claim related to CHB diagnosis identified during the enrolment period) until the earliest of health plan disenrollment or the end of the observation period (June 30, 2020) (Supplementary Fig. 1).
Outcome Measures
Prevalence
The 10-year (2010–2019) prevalence of CHB in the JMDC was calculated as the total number of patients meeting CHB case definition with an index date falling during this period divided by the average JMDC population size during this period. Age- and sex-specific prevalences were also estimated where the JMDC average population size by each age group (< 18, 18–34, 35–44, 45–54, 55–64, 65–74) and sex were used as denominators.
Baseline Variables
Baseline variables were analyzed for eligible patients of the incident cohort. Demographic data included age and sex at baseline. Comorbidities of interest in this study were hypertension, diabetes, hyperlipidemia, peptic ulcer, chronic kidney disease and arthrosis. The Charlson Comorbidity Index (CCI) score, a clinical comorbidity index used with administrative databases based on the presence of 17 comorbidities and their severity, was also calculated; the higher the score, the greater is the disease burden [11] (Supplementary Table 4). In this study, all diseases included in the CCI score were considered in the score calculation except for human immunodeficiency virus (HIV) data, which are not captured by the database. Also, we chose to exclude the CHB disease code from the score calculation since it defines the main pathology of the study sample and not a comorbidity. All comorbidities were defined by the presence of at least one inpatient or outpatient diagnose code during the 12 months prior to index date.
Treatment Patterns
Prescriptions for CHB medications were identified during the observation period for eligible patients in the incident cohort. CHB medication regimens selected for this study were ETV, lamivudine (LAM), TDF, TAF and peg-IFN α-2a. An algorithm was developed to define treatment lines based on several assumptions: (1) All drugs administered within 30 days of line initiation were considered a part of the same regimen and line of therapy. (2) A new line of therapy was defined as either a new drug added after 30 days of initiation or a 90-day gap between two different prescriptions (whichever came first) advancing the line of therapy. The 90-day gap of prescription defined the discontinuation of the previous drug(s). (3) Two consecutive lines containing the same drug(s) (i.e., regimen discontinued and started again after a 90-day gap) were considered as only one line (Supplementary Fig. 2). The maximum number of lines analyzed was three.
Health States
Eligible patients of the incident cohort were categorized into the following health states: CHB, compensated cirrhosis (CC), decompensated cirrhosis (DC) and HCC, using definitions adapted from Oh et al.'s study based on algorithms including ICD-10 codes and/or treatment codes [12] (see Supplementary Table 5). Patients diagnosed with “CHB health state” referred to those diagnosed with CHB without cirrhosis or HCC (at the time of the initial CHB diagnosis). When more than one diagnosis code existed with the same date, the patient was assigned to the most severe health state, being HCC followed by DC and CC. Patients were considered to have CC if they had a diagnosis of liver cirrhosis or esophageal varices without bleeding. Whereas decompensated cirrhotic patients were those diagnosed with any of the following conditions: esophageal varices with bleeding, ascites, hepatic encephalopathy or hepatorenal syndrome. Patients were included in more than one health state if they transitioned from one health state to another over time.
Resource Utilization
HCRU-related outcomes were evaluated for eligible patients of the incident cohort on the period covered by each health state, i.e., from the first event for a particular health state until the first event for the next health state, or the end of follow-up. HCRU considered in this analysis (hospitalizations, pharmacy claims, laboratory testing and outpatient physician visits) were calculated overall and by setting of care, inpatient and outpatient. HCRU and costs were measured per person per year (PPPY) to account for different follow-up periods. Monetary values are presented in Japanese yen (JPY) with value equivalent to US dollar (USD) by applying the average official exchange rate in 2010–2020 as 111.91 JPY = 1 USD in brackets [13].
Statistical Analysis
All study variables were examined descriptively. Categorical variables are shown as numbers (%), and continuous parameters are shown as the means (SDs) or medians (IQRs). The detailed codes and definitions used for this analysis are included in Supplementary Tables 1, 2 and 3. All the analyses were performed using SAS 9.4.
Results
Prevalence
Of 4.1 million beneficiaries in the JMDC claims database during the period of 2010–2019, 13,061 met the CHB case definition, yielding a crude 10-year prevalence of 0.32%. The prevalence of CHB increased with age, with the highest age-specific prevalence (0.95%) reported among adults 65–74 years old. The sex-specific prevalence showed a higher prevalence in men than women (0.36% vs. 0.27%) (Table 1).
Patients’ Characteristics and Comorbidity Burden
A total of 1973 were identified as newly diagnosed CHB patients between 2010 and 2018. Baseline characteristics are described in Table 2. Most of the patients (n = 1827; 92.6%) were > 35 years old; median age at first observed diagnosis was 52 years; 63.7% were men. The average CCI score was 1.92 (SD 2.56), and 64.2% had at least one of the 16 comorbidities defining the CCI. CHB patients showed a high prevalence of comorbidities: hypertension (25.3%), hyperlipidemia (24.2%) and diabetes (22.6%).
Treatment Patterns
The median observation period from first observed CHB diagnosis was 3.1 years (IQR 1.9–5.3, maximum 10.4), during which 295 out of 1973 patients (15%) received at least one prescription for a CHB medication with a predominance of NAs (n = 290/295). Among the 295 patients, the most used drugs were ETV (n = 204, 69.2%) followed by TAF (n = 84, 28.5%). The use of peg-IFN α-2a was less frequent (n = 19, 6.4%) (Table 3). The mean total duration of treatment with ETV was 2.6 years, with TDF 1.8 years and TAF 1.5 years.
Of the 295 patients in first line (1L), most (n = 243; 82.4%) received only one line of therapy through the end of their observation period, 52 (18%) advanced to second line (2L) and 15 (5%) to third line (3L). The most prescribed medications in 1L were ETV (66%) followed by TAF (15%) and TDF (13%). Of the 52 patients in 2L, more than half [n = 28 (53.8%)] received TAF. When looking at the switches from first to second line, most of the patients who started ETV continued the same treatment; the rest switched mainly to TAF or TDF (Fig. 2).
Health States
Among the 1973 newly diagnosed CHB patients and during the 3.1-year median observation period, 68 (3.4%) patients developed and met the inclusion criteria for the CC health state, 29 (1.5%) for DC and 59 (3.0%) for HCC. A considerable number of these complications occurred at the initial diagnosis of CHB (52.9% of CC cases, 27.6% of DC and 44.1% of HCC).
Resource Utilization and Costs
The annual resource utilization of patients in each of the health states is summarized in Table 4. Around 43% of CHB patients had at least one inpatient admission (due to any cause). The annual number of hospitalizations per patient increased as the severity of the disease progressed. Treatment for CHB also increased with disease progression, with the highest rate (52.5%) recorded for HCC patients. Outpatient physician visits were very frequent reaching 11 visit PPPY. Among the total CHB patients in this analysis, the most performed HBV test was HBeAg (58.1%), and 95% were monitored for liver diseases with a frequency of 3.7 screening PPPY (Supplementary Tables 6, 7).
The total all-cause healthcare annual cost was on average 870,568 JPY (7779 USD) for a CHB patient. The estimate of the average annual cost was greatest for the DC health state [3,167,082 JPY (28,300 USD)] and lowest for the CC health state [661,922 JPY (5915 USD)]. Inpatient costs represented a larger component of the total healthcare costs compared to outpatient costs in patients with progressed disease (DC or HCC) (Table 5)
Discussion
This large study aims to further characterize CHB patients, assessing their clinical profiles and management as well as understanding the clinical and economic burden associated with CHB in Japan.
The prevalence of CHB obtained from this study (0.32%) using 2010–2019 data can be considered a reflection of the prevalence of this disease in the last year (2019). Notably, it is lower than that estimated in the 2016 Polaris modeled study (0.6%) [2]. This discrepancy could be attributed to the nature of the database. The JMDC covers 6% of the Japanese population and older age groups (> 75 years), and unemployed individuals are under-represented [9, 10]. The highest prevalence in this study was among adults 65–74 and 55–64 years old (0.95% and 0.86%) and the lowest among children (< 18 years) (0.01%). This finding is expected owing to the effect of national acts to prevent vertical transmission, which essentially eliminated HBV infections in patients < 30 years old.
The mean age found in this study (51.1 ± 11.4) is comparable to that found in US (51.8 ± 12.4 years in 2015), Korean (52.3 ± 12.5 years in 2016) and Hong Kong studies (50.6 ± 16.0 years in 2010–2013 and 54.5 ± 14.9 years in 2014–2017) [12, 14, 15]. For common comorbidities, the prevalence of hypertension in the CHB population (25.3%) was comparable to age-adjusted values from a 2018 National Health Survey (28.0% in men and 19% in women) [16]. However, diabetes and hyperlipidemia were more common in CHB patients than in the survey population despite the age limit of the former (< 75 years) (diabetes: 22.6% versus 6.8–13.5%; hyperlipidemia: 24.2% versus 12.2–18.8%, respectively) [16], highlighting a high frequency of comorbidities in this population.
During the observation period, 15% of the patients have received at least one prescription for a CHB medication, a rate that is considered low compared to 32% treatment eligibility in the country according to 2016 estimations [2]. Currently in Japan, as per 2019 JSH guidelines [4], treatment is indicated in CHB non-cirrhotic patients with an HBV DNA level > 2000 IU/ml and alanine aminotransferase (ALT) > 31 U/l and in cirrhotic patients with positive DNA. The low treatment rate observed in our study may be due to the predominance of asymptomatic carriers in the immune tolerance phase (where ALT levels are within the normal range) or inactive carriers not eligible for treatment. In effect, the incident cohort included only patients with no history of CHB diagnosis, a criterion that must have led to an over-representation of patients belonging to these two phases. Surprisingly, treatment rate was also low in patients with progressed disease (19% in CC and 24% in DC). Another assumption could be that these patients are not yet eligible for treatment because of their negative DNA. Until today, there is insufficient evidence to initiate antiviral therapy in patients with significant fibrosis, but normal or low ALT levels or low DNA levels. This group of patients is not uncommon, and the experts deliberated on the treatment options for them [17]. Unfortunately, laboratory tests results are not available in the JMDC, limiting our capability to support these hypotheses. This low frequency of treatment, which appears to be related to delayed diagnosis, is a common finding across similar studies and an area for improvement in patients’ outcomes. Indeed, in the present study, of the patients who had liver complications, a considerable number occurred at the initial diagnosis of CHB (52.9% of CC cases and 44.1% of HCC cases). These results may show that some patients are not diagnosed with CHB until their disease has progressed and complications begun. Finally, because the definition of drug prescription is based on insurance data, patients who do not meet the criteria for Nas therapy, and who may be on non-reimbursed (out-of-pocket) treatment, are not captured, although this is assumed to be limited given the treatment guideline and duration of treatment (lifetime treatments). Furthermore, the treatment for individuals whose CHB progressed towards HCC or DC could be covered by a special subsidy program implemented from 2018 [18].
In this study, ETV, TDF and TAF were the most prescribed drugs in first line, consistent with JSH current guidelines, due to their low risk of resistance [4]. However, it should be recalled that the definition of first-line treatment was based on several assumptions; in addition, we did not verify whether the patient had started the treatment before inclusion in the study, which could lead to misinterpretation of the results. Although TAF is biing prescribed more often in Japanese clinics currently, the proportion of TAF found in the study was low given its recent approval (2017). Finally, although the reasons for discontinuation or switch are unknown, switching from ETV or TDF to TAF was not surprising as the latter is associated with both improvement of medication compliance and better long-term safety [4, 19].
As expected, the demographics (old age) and comorbidities seen within the CHB patients appear to have translated into high healthcare resource utilization and costs. The results indicate that the liver disease sequelae of CHB have significant economic consequences, as DC and HCC both resulted in high management costs, a phenomenon that was already demonstrated elsewhere [3, 5,6,7, 20]. In the present study, the total healthcare costs of CHB were lower than those estimated in the Yotsuyanagi et al. study [870,568 JPY (7779 USD) vs. 1,332,417 JPY (11,906 USD) PPPY respectively] [3]. These differences could be explained by the lower age and comorbidity burden within our patients compared to those in Yotsuyanagi et al.'s study (mean age = 51.1, mean CCI = 1.9 vs. mean age = 65.2 and mean CCI = 4.8, respectively). Lastly, as with many serious diseases, hospitalizations (inpatient costs) were the economic driver in most of these health states.
This study used real-life data from the JMDC database, which, unlike other types of claims databases that are restricted to hospital data, allowed us to track patients longitudinally across different medical institutions. The JMDC was used in various types of research studies including CHB infection among patients with liver-related diseases [21,22,23]. In addition, the large sample size and long follow-up are important strengths of this study.
However, the findings of this study should be considered within the context of several limitations besides the ones mentioned earlier. While a sizable proportion of CHB patients are elderly over 75 years of age, the limited representation of patients aged > 75 years in the database could reduce the generalizability of findings to the Japanese older population. Despite age being a major limitation of the JMDC, it has been used as a main database for some research studies among elderly Japanese patients [24, 25]. In a study published in 2020, authors showed that the results from the JMDC were comparable to data from the national database [26].
To date, several studies have been performed on the epidemiology and economic burden of CHB in Japan [27,28,29], but we observed a considerable gap in this research area which needs the most recent and updated findings. Therefore, our study provides important evidence, i.e., incidence of CHB at least in the working generation. Incidence of CHB in our study is mostly found to be more prevalent among individuals aged 40–60 years than in other countries. This age range was adequately covered within our study using the JMDC as a data source [30,31,32,33,34]. In addition, a recent publication comparing the JMDC with the National Database of Health Insurance Claims and other publicly available data highlighted the similar distributions of these data sources [10].
Information on laboratory data is very limited in the JMDC, restricting our ability to validate the CHB diagnosis and to identify the phase of the disease based on tests results such as ALT level and HBV viral markers. However, to limit the inclusion of unconfirmed diagnosis, we included only those without suspicious flags in the database. Furthermore, incident case ascertainment was based on a 2-year period before the index during which a history of the disease was identified. Nevertheless, this period is deemed short to detect a history for a chronic disease that does not require regular care, especially in the early stages. Finally, HIV-related information is not available in the JMDC database for privacy protection as it is deemed sensitive information.
Conclusions
This is a new real-world study using reliable data from the JMDC, addressing a data gap in CHB in Japan. Despite the decrease in prevalence observed in recent years, CHB remains a healthcare challenge due to the morbidity and complications associated with the disease progression and the high economic burden that follows. The findings of this study indicate that the liver disease consequences of CHB have significant economic consequences, with DC and HCC leading to high costs, demonstrating the importance of prevention (vaccine) and treatment. Early treatment at the initial stage of the disease and novel treatment regimens are needed to better prevent and reduce the risk of liver disease progression and minimize associated cost, an aspect that should be further investigated.
References
Global hepatitis report, 2017 [Internet]. https://www.who.int/publications-detail-redirect/global-hepatitis-report-2017. Accessed 14 Jan 2022.
Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403.
Yotsuyanagi H, Kurosaki M, Yatsuhashi H, Lee IH, Ng A, Brooks-Rooney C, et al. Characteristics and healthcare costs in the aging hepatitis B population of Japan: a nationwide real-world analysis. Dig Dis. 2022;40(1):68–77.
Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol Res. 2020;50(8):892–923.
Butler JRG, Pianko S, Korda RJ, Nguyen S, Gow PJ, Roberts SK, et al. The direct cost of managing patients with chronic hepatitis B infection in Australia. J Clin Gastroenterol. 2004;38:S187–92.
Hsieh CR, Kuo CW. Cost of chronic hepatitis B virus infection in Taiwan. J Clin Gastroenterol. 2004;38:S148–52.
Shon C, Choi HY, Shim JJ, Park SY, Lee KS, Yoon SJ, et al. The economic burden of hepatitis A, B, and C in South Korea. Jpn J Infect Dis. 2016;69(1):18–27.
Yang S, Chen G, Li Y, Li G, Liang Y, Zhou F, et al. The trend of direct medical costs and associated factors in patients with chronic hepatitis B in Guangzhou, China: an eight-year retrospective cohort study. BMC Med Inform Decis Mak. 2021;21(S2):71.
Nagai K, Tanaka T, Kodaira N, Kimura S, Takahashi Y, Nakayama T. Data resource profile: JMDC claims databases sourced from Medical Institutions. J Gen Fam Med. 2020;21(6):211–8.
Nagai K, Tanaka T, Kodaira N, Kimura S, Takahashi Y, Nakayama T. Data resource profile: JMDC claims database sourced from health insurance societies. J Gen Fam Med. 2021;22(3):118–27.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–83.
Oh H, Jun DW, Lee IH, Ahn HJ, Kim BO, Jung S, et al. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment Pharmacol Ther. 2020;52(2):371–81.
Official exchange rate (LCU per US$, period average) - Japan | Data [Internet]. 2022. https://data.worldbank.org/indicator/PA.NUS.FCRF?end=2020&locations=JP&start=2010.
Nguyen MH, Lim JK, BurakOzbay A, Fraysse J, Liou I, Meyer N, et al. Advancing age and comorbidity in a US insured population-based cohort of patients with chronic hepatitis B: hepatology. Hepatology. 2019;69(3):959–73.
Wong GL, Wong VW, Yuen BW, Tse Y, Luk HW, Yip TC, et al. An aging population of chronic hepatitis b with increasing comorbidities: a territory-wide study from 2000 to 2017. Hepatology. 2020;71(2):444–55.
The National Health and Nutrition Survey (NHNS) Japan, 2018 [Internet]. https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2018.pdf. Accessed 22 Apr 2022.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Kanto T. Messages from Japan policy for viral hepatitis. Glob Health Med. 2021;3(5):249–52.
Uchida Y, Nakao M, Tsuji S, Uemura H, Kouyama J, Naiki K, et al. Significance of switching of the nucleos(t)ide analog used to treat Japanese patients with chronic hepatitis B virus infection from entecavir to tenofovir alafenamide fumarate. J Med Virol. 2020;92(3):329–38.
Lee TA, Veenstra DL, Iloeje UH, Sullivan SD. Cost of chronic hepatitis B infection in the United States. J Clin Gastroenterol. 2004;38:S144–7.
Hirata A, Hirata T, Takahashi Y, Nakayama T. Surveillance rates for hepatocellular carcinoma among patients with cirrhosis, chronic hepatitis B, and chronic hepatitis C based on Japanese claims database. Hepatol Res. 2017;47(4):283–92.
Ohisa M, Kimura Y, Matsuo J, Akita T, Sato T, Matsuoka T, et al. Estimated numbers of patients with liver disease related to hepatitis B or C virus infection based on the database reconstructed from medical claims from 2008 to 2010 in Japan. Hepatol Res. 2015;45(12):1228–40.
Ikeda M, Yamamoto H, Kaneko M, Oshima H, Takahashi H, Umemoto K, et al. Screening rate for hepatitis B virus infection in patients undergoing chemotherapy in Japan. Int J Clin Oncol. 2016;21(6):1162–6.
Fukushima K, Mizuoka S, Yamamoto S, Iizuka T. Patient cost sharing and medical expenditures for the Elderly. J Health Econ. 2016;45:115–30.
Akazawa M, Imai H, Igarashi A, Tsutani K. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8(2):146–60.
Ishii T, Shiota S, Yamamoto K, Abe K, Miyazaki E. Inhaled corticosteroid-containing regimens reduce hospitalizations and healthcare costs among elderly asthmatics: real-world validation using the national health insurance claims database. Tohoku J Exp Med. 2020;251(2):135–45.
Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15(12):1356–61.
Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15(Suppl):E3-6.
Tanaka J, Kurisu A, Ohara M, Ouoba S, Ohisa M, Sugiyama A, et al. Burden of chronic hepatitis B and C infections in 2015 and future trends in Japan: a simulation study. Lancet Reg Health West Pac. 2022;22: 100428.
Lu M, Zhou Y, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, et al. Trends in diagnosed chronic hepatitis B in a US health system population, 2006–2015. Open Forum Infect Dis. 2019;6(7):ofz286.
Hu YC, Yeh CC, Chen RY, Su CT, Wang WC, Bai CH, et al. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ. 2018;6: e4297.
Liu A, Le A, Zhang J, Wong C, Wong C, Henry L, et al. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000–2015. Clin Transl Gastroenterol. 2018;9(3):141.
Yuen MF, Yuan HJ, Wong DKH, Yuen JCH, Wong WM, Chan AOO, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54(11):1610–4.
Basyigit S, Sapmaz F. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: a 10-year follow-up of 673 patients. Eur J Gastroenterol Hepatol. 2015;27(10):1230.
Acknowledgements
Funding
This study, including the journal’s Rapid Service fee, was funded by Janssen Asia Pacific. Cerner Enviza received funding from Janssen Asia Pacific during the conduct of the study and has no other funding, financial relationships or conflict of interest to disclose.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Kittima Wattanakamolkul conceived the idea. Takeji Umemura, David Bin-Chia Wu, Kittima Wattanakamolkul, Yoshikazu Nakayama, Yasushi Takahashi, Urbano Sbarigia, Lim KyungHwa and Angelina Villasis-Keever contributed to the design of the study, analysis and interpretation of data and drafting the article. Martina Furegato, Jessica Azzi, Laurene Gauthier and Gregoire Nowacki contributed to the acquisition of data and revising the article. Takeji Umemura, David Bin-Chia Wu, Kittima Wattanakamolkul, Yoshikazu Nakayama, Yasushi Takahashi, Urbano Sbarigia, Angelina Villasis-Keeverand and Jessica Azzi participated in the analysis and interpretation of data and revision of the article. All authors gave their final approval of the version to be submitted.
Disclosures
Takeji Umemura has nothing to disclose. Martina Furegato, Laurène Gautier, Grégoire Nowacki and Jessica Azzi are employees of Cerner Enviza and have nothing to disclose. Kittima Wattanakamolkul, Yoshikazu Nakayama, Yasushi Takahashi, Urbano Sbarigia, Lim KyungHwa, Angelina Villasis-Keever and David Bin-Chia Wu are employees of Janssen and own stock of Johnson & Johnson.
Prior Presentation
Poster presentation at JSH2022.
Compliance with Ethics Guidelines
The study utilized de-identified data from the JMDC Claims database. Based on Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labour and Welfare, ethics approval was not applicable to this study.
Data Availability
Given the administrative nature of the data, patients did not provide informed consent for data sharing; however, all data are fully anonymized, and the risk of patient identification is low.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Umemura, T., Wattanakamolkul, K., Nakayama, Y. et al. Real-World Epidemiology, Clinical and Economic Burden of Chronic Hepatitis B in Japan: A Retrospective Study Using JMDC Claims Database. Infect Dis Ther 12, 1337–1349 (2023). https://doi.org/10.1007/s40121-023-00795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00795-0