Abstract
Introduction
Evidence shows that treatment for hepatitis B virus (HBV) can suppress viral load. Among the factors directly linked to therapeutic success is adherence to the treatment. Several instruments to assess adherence are available, but they are not validated for use in chronic hepatitis B. The purpose of this paper was to adapt and validate the “Assessment of Adherence to Antiretroviral Therapy Questionnaire—HIV” (CEAT-VIH) for patients with chronic hepatitis B (referred to herein as CEAT-HBV).
Methods
The validity of the adapted questionnaire evidence was established through concurrent, criterion, and construct validities.
Results
We found negative and significant correlation between the domain “degree of compliance to antiviral therapy” assessed by CEAT-HBV and the Morisky test (r = −0.62, P < 0.001) and between the domain “barriers to adherence” and HBV viral load (r = −0.42, P < 0.001). In terms of the construct’s discriminative capacity, scores greater than or equal to 80 detected antiviral therapy success, which are necessary for the prediction of an undetectable HBV viral load. Thus, a cutoff value of 80.5 was set with a value of 81% for sensitivity and 67% for specificity.
Conclusion
The CEAT-HBV identified 43% (n = 79) non-adherent patients and was shown to be a useful tool in clinical practice.
Similar content being viewed by others
Introduction
Chronic infection caused by hepatitis B virus (HBV) is an important public health issue [1, 2]. Worldwide it is estimated 240 million people are chronically infected [3]. In Brazil, 5441 deaths were reported during the period from 2000 to 2009, with a median of 527.5 deaths per year and an approximate death rate of 0.3–0.4 per 100,000 habitants [4].
The main objectives of hepatitis B treatment are to reduce the progression of the hepatic damage and to eliminate HBV, which minimizes the conversion to cirrhosis and the development of hepatocellular carcinoma. Since several patients do not achieve a sustained viral response, treatment will usually last many years, which increases the probability of selecting resistant viral strains, and consequently the therapeutic options will be reduced [5, 6].
Factors such as viral mutations, the reduced genetic barrier of certain drugs, and the lack of adherence to antiviral therapy contribute to drug resistance [7–13]. A few authors have pointed to adherence to antiviral therapy as a key point in therapeutic success, which reduces drug resistance, HBV viral load, and the cost of treatment [5, 11, 14–16].
Sogni and collaborators demonstrated that therapeutic education and a systematic assessment of drug therapy adherence using self-reporting should be promoted to ensure the efficacy of a long-term treatment [17]. Thus, structured questionnaires are the first choice due to their easy application and low cost [18].
Several self-reported measures for the assessment of drug treatment adherence are available. Many instruments are generic in their scope, such as the Morisky test [18], and others are only a subjective clinical evaluation by a health professional [19]. A few address adherence from a disease-specific perspective [20, 21, 25, 29]. However, at the time this study was initiated, a validated questionnaire for assessing antiviral therapy adherence specific for chronic hepatitis B was not found in the literature. As studying the validation of a specific instrument for this patient group is essential before investigating probable causes of non-adherence, we proposed adapting the “Assessment of Adherence to Antiretroviral Therapy Questionnaire—HIV” (CEAT-VIH) to assess adherence in HBV-infected patients.
The most important parameter in HBV treatment is the viral load; however, it is dependent on a specific laboratory, trained staff, and equipment. On the other hand, questionnaires about drug therapy adherence are low cost and give an immediate answer to doctors; however, validated questionnaires regarding adherence to anti-HBV drugs do not exist. In this context, this work aimed to describe the adaptation of the CEAT-VIH questionnaire, developed for assessing adherence by patients taking HIV antiretroviral treatment [20, 21]. The option for this questionnaire was based on the version in Portuguese, which simplified the process of adaptation, since the transcultural and translation for the Brazilian culture were already done. The original version of this questionnaire for HIV has been applied by researchers and health care professionals since 1999 [21].
Methods
This was a validation study. The target population was patients with chronic infection with HBV treated in a public tertiary hospital reference center. The period of study was December 2010 to August 2011, and the sampling was consecutive (not probabilistic). This research project was approved by the Institutional Review Board of the Hospital das Clínicas of the University of Sao Paulo School of Medicine (protocol 0581/10).
Patients
Inclusion criteria were: both sexes; age ≥18 years; clinical diagnosis of chronic hepatitis B; use of at least 3 months of one or more anti-HBV antivirals (i.e., adefovir, entecavir, lamivudine, and tenofovir); willingness and capacity to answer the questionnaire; ability to provide written informed consent; and ability to return for scheduled treatment and assessment. Exclusion criteria were: diagnosis of chronic hepatitis C virus and/or HIV infection.
Each patient was evaluated during one medical appointment. The protocol consisted of application of the Morisky test and the CEAT-HBV. A blood sample for HBV viral load determination was collected on the same day. HBV viral load was determined by the COBAS AmpliPrep-COBAS TaqMan HBV test (CAP-CTM; Roche Molecular Systems, Inc., Branchburg, NJ, USA). The HBV viral load detection limit was between 54.5 and 110,000,000 IU/mL. The viral load determination was used in the CEAT-HBV validation. Socio-demographic data (gender, age, race, and education), hepatitis B information, and the patient’s perception about the antiviral therapy were also collected. Clinical profile data were obtained from the physician registration form: HBV viral load, antiviral drugs in use, and duration of treatment. All the patients were examined by two hepatologists and a pharmacist applied the research questionnaires.
Instrument
The instrument was the validated CEAT-VIH questionnaire, Portuguese (Brazilian) version, an instrument with twenty questions that intends to assess the level of patient adherence to antiretroviral therapy [20].
Since the Portuguese version of CEAT-VIH has been shown to be an adequate and effective tool to verify the level of antiretroviral therapy adherence in patients with HIV [21], we proposed to validate this instrument for patients with chronic hepatitis B, who are also subject to viral resistance. The adapted version was called the “Assessment of Adherence to Antiviral Therapy Questionnaire” (CEAT-HBV).
As a step in the adaptation process of the CEAT-VIH for the CEAT-HBV, the word “HIV” was replaced by “hepatitis B” in items 8, 10, 15, and 17. In the other items neither the questions nor the options for answers were modified [20]. Then, the modified version was reviewed by the two hepatologists who concluded that this version could be applied to assess anti-HBV therapy adherence.
The adapted version, CEAT-HBV, had 20 questions and was divided into two domains. One domain was called “degree of compliance with antiviral therapy” with five questions (1–4 and 12). The other domain was called “barriers to adherence” with the other fifteen questions (5–11 and 13–20) [22, 23].
The answers to the questions use the 5-point Likert scale (a higher score indicating greater adherence to the treatment) except questions 5, 19, and 20. On question 5, the score varies from zero to two: zero indicates patients who did not remember the name and dosage of the antiviral administered; one point for those who knew only the name or dosage and two points for those who knew both the name and dosage of the antiviral. On questions 19 and 20, the score can be zero or one (a negative answer on question 19 and an affirmative answer on question 20 scored one). The full questionnaire ranges from 17 to 89 points.
Since there is no gold standard assessment of antiviral drug adherence, we adopted the Morisky test [18] to identify the level of antiviral therapy adherence, from a generic measure perspective. The original Morisky test has four items that have dichotomous response categories with yes or no. The rationale behind the four items was “drug errors of omission could occur in any or all of several ways: forgetting, carelessness, stopping the drug when feeling better or starting the drug when feeling worse” [18].
Statistical Analysis
Data were described using mean, standard deviation, median, minimum and maximum values and frequency distribution. The Q Cochran test was used to compare the level of adherence between the CEAT-HBV, the Morisky test and clinical outcome (HBV viral load detectable/undetectable). To evaluate the time of treatment with antiviral drugs (in months), patients were classified according to clinical outcome and adherence to antiviral drug treatment and the t Student test for independent samples was applied. To verify the correlation between the HBV viral load and time of antiviral drug treatment, the Pearson coefficient of correlation was calculated. The questionnaire reliability was verified using Cronbach’s alpha coefficient [24]. The construct validation of the CEAT-HBV was established using concurrent and criterion validities.
The convergent validation of criterion and construct was evaluated by a Spearman correlation between the score on each domain of the questionnaire (antiviral drug treatment compliance and barriers to non-adherence) and the score on the Morisky test and HBV viral load, respectively. The correlation between the total score on the CEAT-HBV, the Morisky test, and HBV viral load was also calculated.
The discriminative capacity was evaluated to verify if each domain and the full questionnaire were sensitive to distinguishing the clinical outcome, i.e., patients with undetectable HBV viral load. To do this, patients were classified according to HBV viral load (detectable and undetectable) in the last 6 months and the scores for each domain and of the whole questionnaire were compared using the Mann–Whitney test. Data were expressed as median and interquartile range (IQR).
Content validity was determined at the moment of design of the original questionnaire, the CEAT-VIH, and was based on the theoretical model of the instrument [20].
A receiver operating characteristic (ROC) curve determined the sensibility and specificity of the CEAT-HBV, and patients were classified according to HBV viral load (detectable or undetectable).
Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and SPSS version 13.0 (IBM Corporation, Armonk, NY, USA) were used for statistical analyses. The significance level was set at 0.05.
Results
We screened 580 patients and 230 patients were registered as taking any antiviral drug for HBV treatment in the hospital pharmacy. After applying the inclusion criteria, 183 patients fulfilled it and comprised the sample in this study (Fig. 1).
Socio-demographic data on the patients are depicted in Table 1. Regarding antiviral therapy, 53.6% (n = 98) of patients received lamivudine as monotherapy, 3.3% (n = 6) received adefovir as monotherapy, 10.9% (n = 20) received tenofovir as monotherapy, 15.3% (n = 28) received lamivudine and adefovir, and 10.4% (n = 19) received lamivudine and tenofovir.
The CEAT-HBV presented satisfactory acceptance as a structured clinical interview. The minimum and maximum scores were 50 and 89, respectively, and the total median score was 80 (IQR: 77–83). A floor effect was not observed and the ceiling effect was 0.5% (percentage of subjects who scored the minimum and maximum possible score in the questionnaire; some authors have recommended that it should be less than 20%) [21, 25].
The reliability for the total questionnaire (20 items, α = 0.73) and in the domain “degree of compliance with antiviral therapy” (5 items, α = 0.83) was satisfactory. However, the reliability of the domain “barriers to adherence” was less than expected (15 items, α = 0.66), but was still acceptable.
Construct validity assessed by a concurrent method showed that the domain “degree of compliance with antiviral therapy” presented a moderate and negative correlation with the Morisky test score (r = −0.62, P < 0.001) and the domain “barriers to adherence” presented a moderate and negative correlation with HBV viral load (r = −0.42, P < 0.001). The total score of the CEAT-HBV, indicating global adherence, also presented a moderate and negative correlation with the Morisky test (r = −0.44, P < 0.001) and with the HBV viral load (r = −0.47, P < 0.001).
The discriminative capacity of the questionnaire was verified with a comparison of the scores on the questionnaire (global score and each domain) that were statistically different concerning the clinical outcome (P < 0.001; Table 2). There was no intersection between the IQRs of the CEAT-HBV score among patients with or without HBV viral load (P < 0.001). Based on this observation, we established a score of 80 to discriminate adherent from non-adherent patients.
Assessing the duration of treatment as a relevant bias for adherence was checked in patients with (64.1 ± 54.8 months) and without (70.0 ± 48.3 months) HBV viral load (P = 0.118). The treatment duration of adherent patients (determined by a score of 80 on the CEAT-HBV) was 73.6 ± 50.5 months and of non-adherent patients 60.0 ± 50.5 months (P = 0.607). The HBV viral load did not correlate with antiviral treatment duration (r = −0.06, P = 0.456).
The CEAT-HBV found 43.2% (n = 79) of patients were non-adherent. The Morisky test found 46.4% (n = 85) non-adherent patients and HBV viral load identified 38.3% (n = 70) non-adherent patients, without differences between the methods (P = 0.143).
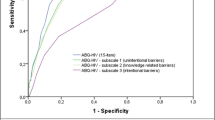
The ROC curve (Fig. 2) for the CEAT-HBV demonstrated the capacity of the questionnaire in classifying adherent and non-adherent patients (P < 0.001). We present the sensibility and specificity of the curve (Table 3) and set the cutoff at 80.50, which was associated with a sensibility of 81.4% and a specificity of 67.3%.
Receiver operating characteristic curve of the CEAT-HBV and sensibility and specificity indicators. For the cutoff of 80.50, a sensibility of 81% and specificity of 67%. Area under the curve: 80%, P < 0.001. Data source: Hospital das Clínicas of the University of Sao Paulo School of Medicine, December 2010 to August 2011 (n = 183)
Discussion
In the present study, we tested the reliability of the CEAT-HBV in patients with HBV chronic infection using different psychometric markers. The questionnaire presented a satisfactory result according to parameters established in the literature [24].
When the hypothesis of the multi-dimensionality of the questionnaire was tested with the division of the instrument into two domains “degree of compliance with antiviral therapy” and “barriers to adherence”, we verified that the second domain presented a less than expected internal consistency. Therefore, the complete questionnaire should always be used to maintain the psychometric properties related to reliability.
Both the domain “degree of compliance with antiviral therapy” and the global adherence score of the CEAT-HBV presented reliabilities (α = 0.83 and α = 0.71, respectively) higher than that of the Morisky test (α = 0.61), which is an advantage in using CEAT-HBV instead of the Morisky test.
We hypothesized two reasons for the domain “barriers to adherence” presenting an internal consistency less than 0.70. First, the pattern of answers in the domain for the current sample showed a non-normal distribution (i.e., asymmetric and leptokurtic distribution) that can affect the reliability coefficient. Second, the fifteen questions included in this domain could be harboring more dimensions than proposed [22], such as the doctor–patient relationship, collateral effects, perception of the infection, and others. Future studies can explore this hypothesis.
An analysis of the reproducibility of a scale was not performed because the questionnaire was applied only once to each patient.
Adapting a specific instrument for patients who live with HIV [20, 26] to the situation of chronic HBV carriers was proposed initially. The choice of instrument proposed by Remor and collaborators [20] was due to its availability in Portuguese, its validation and content of the questions of interest, making the unnecessary translation and cultural adaptation of other instruments redundant. We considered both the similarities between these treatments and the differences, such as the stigma and psychological impact, thus justifying the necessity of the adaptation and validation before application of the CEAT-HBV [27, 28].
As expected, the domains of the CEAT-HBV presented acceptable construct validity, assessed by criterion-related and convergent methods, since there was statistical correlation with established measures considered gold standards for these constructs (i.e., the Morisky test score and HBV viral load).
The analysis of the construct’s discriminative capacity can be considered satisfactory, since the total score on the questionnaire was sufficient to classify patients according to their viral load level, that is, detectable/undetectable viral load (P < 0.001). However, in the discriminative capacity of the domains, we observed an intersection between the IQRs that showed the necessity of considering the global score of the questionnaire to evaluate adherence to the antiviral therapy, as happens with the original CEAT-VIH [20, 26].
Moreover, we could set a cutoff point on the global score questionnaire: a score of less than 80 points indicates patients who did not adhere to antiviral therapy and usually had a detectable HBV viral load detectable. On the other hand, a score greater than or equal to 80 points indicates patients who adhered to the antiviral therapy and usually have an undetectable HBV viral load.
The CEAT-HBV found 43.2% (n = 79) of patients were non-adherent. The overview of adherence studies in hepatitis B showed that the frequency of adherent patients was between 35 and 74% [16, 29].
The duration of the antiviral therapy could compromise the validation of the questionnaire if there were differences between the mean duration treatment, classified according to the clinical outcome (HBV viral load detectable or undetectable) and adherence to treatment (or non-adherence). This fact could be explained by the duration needed (generally up to a year) for patients to present an undetectable HBV viral load. However, as all the groups presented a treatment duration longer than 12 months with a lack of statistical differences, we can state that these two clinical variables did not compromise the validation of the questionnaire. The lack of correlation (r = −0.06, P = 0.456) between HBV viral load and time of treatment reinforces this statement.
Patients who were using alpha-interferon or pegylated interferon were excluded because this treatment is administered for a limited period of time and the collateral effects were superior to those of the antiviral drugs, which would compromise the questionnaire validation.
The Morisky test presented low reliability in the present study and poor discriminative capacity for clinically related markers. So, this test identifies many false positives, which is not unacceptable in a study of adherence to antiviral therapy due to drug resistance [14, 18]. In contrast, the CEAT-HBV was shown to be a simple diagnostic tool, useful and easy, and its use should be widespread in the hepatology area. As the questionnaire was validated using the viral load of HBV as the gold standard, regions with limited financial resources could use the questionnaire for the early prediction of outcome. Furthermore, in the present study we verified that the CEAT-HBV presented greater sensibility and specificity in comparison to those reported for the Morisky test (81 and 44%, respectively) [18] and the CEAT-HIV (79 and 57%, respectively) [20]. Other studies have highlighted the low discriminative capacity of the Morisky test [30–32].
Poor adherence to antivirals treatment leads to increased risk of drug resistance and treatment failure. The present study measured treatment failure as HBV viral load rebound and whether the treatment failure is only due to adherence or development of resistance remains a question and needs further evaluations.
The response to treatment depends on factors such as adherence, presence of resistance and antiviral potency. Entecavir and tenofovir are more potent antivirals with high genetic barrier to resistance; therefore, it is possible that patients with non-adherence would need longer time to observe treatment failure [15].
In this study, the results showed that an instrument proposed for patients with HIV can be used for patients with chronic HBV. It is noteworthy to mention that the adherence is fully monitored in randomized controlled trials, which brings curiosity about the patients’ behavior concerning adherence in real life, as observed by recent researchers [5, 16, 17].
Conclusions
CEAT-HBV is an instrument with adequate reliability, validity, and discriminative capacity. It is adequate to assess the adherence to antiviral therapy and predict the clinical outcome of the patient (HBV viral load detectability), making it a valuable tool in clinical practice. Furthermore, it is the first specific instrument suitable for the evaluation of antiviral treatment adherence in patients with chronic HBV; however, more studies about the advantages and disadvantages of each instrument should be conducted. At this time, it seems appropriate to recommend the CEAT-HBV as a useful tool for the doctor in clinical practice with patient non-responders to antiviral drug treatment, as a first step toward improving antiviral therapy adherence, which is considered a key factor for therapeutic success.
References
Lacey L. Review of economic benefits of treating chronic hepatitis B with lamivudine. J Gastroenterol Hepatol. 2004;19(Suppl):S10–2.
Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13(Suppl 1):S47–9.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–9.
MeC MeC, Amorim TR, Pereira GF, Araújo WN. Hepatitis B mortality in Brazil, 2000–2009. Cad Saude Publica. 2012;28(3):472–8.
Chotiyaputta W, Peterson C, Ditah FA, Goodwin D, Lok AS. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. J Hepatol. 2011;54(1):12–8.
Kim V, Abreu RM, Nakagawa DM, Baldassare RM, Carrilho FJ, Ono SK. Pegylated interferon alfa for chronic hepatitis B: systematic review and meta-analysis. J Viral Hepat. 2015. doi:10.1111/jvh.12418
Locarnini S, Hatzakis A, Heathcote J, Keeffe EB, Liang TJ, Mutimer D, et al. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9(5):679–93.
Hoofnagle JH. Hepatitis B—preventable and now treatable. N Engl J Med. 2006;354(10):1074–6.
Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4(8):936–62.
Ayoub WS, Keeffe EB. Review article: current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2011;34(10):1145–58.
Ha NB, Garcia RT, Trinh HN, Chaung KT, Nguyen HA, Nguyen KK, et al. Medication nonadherence with long-term management of patients with hepatitis B e antigen-negative chronic hepatitis B. Dig Dis Sci. 2011;56(8):2423–31.
Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, et al. The polymerase L528 M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001;107(4):449–55.
Ono-Nita SK, Kato N, Shiratori Y, Lan KH, Yoshida H, Carrilho FJ, et al. Susceptibility of lamivudine-resistant hepatitis B virus to other reverse transcriptase inhibitors. J Clin Invest. 1999;103(12):1635–40.
Hilleret MN, Larrat S, Stanke-Labesque F, Leroy V. Does adherence to hepatitis B antiviral treatment correlate with virological response and risk of breakthrough? J Hepatol. 2011;55(6):1468–9 (author reply 9–70).
Zoulim F. Hepatitis: treatment failure in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2011;8(7):366–7.
Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19(3):205–12.
Sogni P, Carrieri MP, Fontaine H, Mallet V, Vallet-Pichard A, Trabut JB, et al. The role of adherence in virological suppression in patients receiving anti-HBV analogues. Antivir Ther. 2012;17(2):395–400.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Haynes RB, Taylor DW, Sackett DL, Gibson ES, Bernholz CD, Mukherjee J. Can simple clinical measurements detect patient noncompliance? Hypertension. 1980;2(6):757–64.
Remor E, Milner-Moskovics J, Preussler G. Brazilian adaptation of the assessment of adherence to antiretroviral therapy questionnaire. Rev Saude Publica. 2007;41(5):685–94.
Remor E. Systematic review of the psychometric properties of the questionnaire to evaluate the adherence to HIV therapy (CEAT-VIH). Patient. 2013;6(2):61–73.
Dima AL, Schweitzer AM, Diaconiţ R, Remor E, Wanless RS. Adherence to ARV medication in Romanian young adults: self-reported behaviour and psychological barriers. Psychol Health Med. 2013;18(3):343–54.
Abreu RM. Questionnaire validation for adherence antiviral therapy assessment in chronic hepatitis B patients. São Paulo: University of Sao Paulo School of Medicine; 2013.
Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52(4):281–302.
Mao HF, Hsueh IP, Tang PF, Sheu CF, Hsieh CL. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke. 2002;33(4):1022–7.
Remor E. Valoración de la adhesión al tratamiento antirretroviral en pacientes VIH+. Psicothema. 2002;14(2):262–7.
Brasil. Ministério da Saúde. Protocolo clínico e diretrizes terapêuticas para o tratamento da hepatite viral crônica B e coinfecções. Brasília: Ministério da Saúde; 2010.
Brasil. Ministério da Saúde. Recomendações para Terapia Anti-retroviral em Adultos Infectados pelo HIV. 7 ed. Brasília: Ministério da Saúde; 2008.
Lieveld FI, van Vlerken LG, Siersema PD, van Erpecum KJ. Patient adherence to antiviral treatment for chronic hepatitis B and C: a systematic review. Ann Hepatol. 2013;12(3):380–91.
García-Llana H, Remor E, Selgas R. Adherence to treatment, emotional state and quality of life in patients with end-stage renal disease undergoing dialysis. Psicothema. 2013;25(1):79–86.
Koschack J, Marx G, Schnakenberg J, Kochen MM, Himmel W. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties. J Clin Epidemiol. 2010;63(3):299–306.
Voils CI, Yancy WS. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties. J Clin Epidemiol. 2011;64(3):340–1 (discussion 1–2).
Acknowledgments
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Alves de Queiroz Family Fund for Research. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Authors’ contributions
R. M. Abreu contributed to the concept and design of the study, data collection, analysis and interpretation of the results, wrote the manuscript and the final revision of the article. C. S. Ferreira contributed to the data collection. A. S. Ferreira contributed to the analysis and interpretation of the results and to the final revision of the article. E. Remor contributed to the concept and design of the study, the analysis and interpretation of the results and to the final revision of the article. P. D. Nasser contributed to the data collection. F. J. Carrilho contributed to the concept and design of the study, to the final revision of the article and approved the final version of the article. S. K. Ono contributed to the concept and design of the study, the data collection, analysis and interpretation of the results, and wrote the manuscript and the final revision of the article.
Disclosures
R. M. Abreu, C. S. Ferreira, A. S. Ferreira, E. Remor, P. D. Nasser, F. J. Carrilho, and S. K. Ono have nothing to disclose.
Compliance with Ethics Guidelines
This research project was approved by the Institutional Review Board of the Hospital das Clínicas of the University of Sao Paulo School of Medicine (protocol 0581/10). Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abreu, R.M., da Silva Ferreira, C., Ferreira, A.S. et al. Assessment of Adherence to Prescribed Therapy in Patients with Chronic Hepatitis B. Infect Dis Ther 5, 53–64 (2016). https://doi.org/10.1007/s40121-015-0101-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-015-0101-y