Abstract
Introduction
The inhibition of the calcitonin gene-related peptide (CGRP) pathway has attracted interest in pharmacological research on migraine. Atogepant is a potent, selective, orally available antagonist of the CGRP receptor approved as a preventive treatment of episodic migraine. This systematic review with meta-analysis aims to evaluate the efficacy and safety of atogepant for the prevention of episodic migraine in adult patients.
Methods
Randomized, placebo-controlled, single or double-blinded trials were identified through a systematic literature search (December week 4, 2021). Main outcomes included the changes from baseline in monthly migraine days and the incidence of adverse events (AEs) and treatment withdrawal due to AEs. Mean difference (MD) and risk ratio (RR) with 95% confidence intervals (95% CIs) were estimated.
Results
Two trials were included, overall enrolling 1550 patients. A total of 408 participants were randomized to placebo, 314 to atogepant 10 mg, 411 to atogepant 30 mg, and 417 to atogepant 60 mg once daily. The mean age of the patients was 41.0 years and 87.7% were women. The reduction in the mean number of migraine days from baseline across the 12-week treatment period was significantly greater among patients treated with atogepant at either the daily dose of 10 mg (MD − 1.16, 95% CI − 1.60 to − 0.73, p < 0.001), 30 mg (MD − 1.15, 95% CI − 1.54 to − 0.76, p < 0.001), or 60 mg (MD − 1.20, 95% CI − 2.18 to − 0.22, p = 0.016) than with placebo. There were no differences in the occurrence of AEs and drug withdrawal due to AEs between atogepant and placebo groups. Constipation was more commonly observed in patients treated with atogepant at 30 mg/day than placebo (RR 5.19, 95% CI 2.00–13.46; p = 0.001). Treatment with atogepant at the daily dose of 60 mg was associated with a higher risk of constipation (RR 4.92, 95% CI 1.89–12.79; p = 0.001) and nausea (RR 2.73, 95% CI 1.47–5.06; p = 0.001) than placebo.
Conclusion
Atogepant is an efficacious and overall well-tolerated treatment for the prevention of episodic migraine in adults.
Similar content being viewed by others
The inhibition of the calcitonin gene-related peptide (CGRP) pathway has attracted interest in the pharmacological research on migraine. |
Atogepant is a potent, selective, orally available antagonist of the CGRP receptor. |
Once daily atogepant is an efficacious and overall well-tolerated treatment for the prevention of episodic migraine in adults. |
Introduction
Migraine is one of the most common neurological disorders with an estimated prevalence of 15% and more than one billion people suffering from this condition worldwide [1]. Migraine is characterized by recurrent headache, with each episode lasting 4–72 h. The pain is typically described as pulsating, moderate to severe in intensity, and unilateral. It is accompanied by nausea and/or vomiting, photo- and/or phonophobia, and it is aggravated by routine physical activity [2]. Depending on the frequency of the attacks, migraine is defined as “episodic” when migraine days are up to 14 per month, and “chronic” when headache occurs on 15 or more days per month with the characteristics of migraine on at least 8 days/month [2]. As the symptoms affect work-life, social and leisure activities, physical and emotional functioning, migraine may result in a substantial burden on patients and families and a significant increase in healthcare expenditure [3,4,5,6].
Research revealed that calcitonin gene-related peptide (CGRP) is a vasodilator and neuromodulator that plays a key role in the pathophysiology of migraine. Intravenous infusion of CGRP can induce a migraine-like headache in migraineurs [7, 8], serum levels of CGRP are increased interictally in patients with migraine [9, 10], salivary levels of CGRP are elevated during migraine attacks and are reduced with triptan and botulinum toxin administration [11, 12].
The inhibition of the CGRP pathway represents an effective therapeutic strategy. Four monoclonal antibodies targeting the CGRP receptor (erenumab) or ligand (galcanezumab, fremanezumab, and eptinezumab) are available for the preventive treatment of migraine. Two oral CGRP receptor antagonists (rimegepant and ubrogepant) are approved for the treatment of migraine attacks, and rimegepant received additional approval for migraine prophylaxis in adults.
Atogepant is a potent, selective, orally available antagonist of the CGRP receptor [13]. The drug was approved in September 2021 by the US Food and Drug Administration for the preventive treatment of episodic migraine in adults at the recommended dosages of 10, 30, or 60 mg once daily [13]. This systematic review with meta-analysis aims to provide a comprehensive qualitative and quantitative synthesis of the efficacy and safety of atogepant.
Methods
Search Strategy
This systematic review and meta-analysis is reported according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14]. We systematically searched (December week 4, 2021) MEDLINE (accessed by PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), and the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov); details of the search strategies are outlined in the Supplementary Material. There were no date limitations or language restrictions. The reference lists of retrieved studies were reviewed to identify additional reports of relevant trials. The protocol was not previously registered.
Eligibility Criteria
Studies were selected when they met the following entry criteria: randomized, single or double-blinded, placebo-controlled, parallel-group studies with active and control groups receiving atogepant and matched placebo, respectively. Participants had to meet the following criteria: any sex, adult age (18 years or older), history of migraine, with or without aura, and 4–14 migraine days per month during the baseline period [2].
Outcome Measures
The efficacy outcomes were the changes from baseline in the mean number of migraine days, headache days, and days of use of medication for the treatment of migraine attacks per month and the proportions of participants with a reduction of at least 50% from baseline in the mean number of migraine days per month across the double blind-treatment period. The safety and tolerability outcomes included the proportions of participants who experienced any adverse event (AE), any serious AE (SAE), and withdrew from treatment for AEs. The AEs reported in at least 5% of patients in any group in any trial were further summarized.
Study Selection, Data Extraction, and Assessment of Risk of Bias and Certainty of Evidence
Two review authors (SL and FV) independently assessed trials for inclusion and extracted the following information from included studies: main study author, age of publication, methodology and trial design (methods of randomization, allocation concealment and blinding, duration of baseline and treatment periods, dose(s) of atogepant tested), number, demographics and clinical characteristics of participants (age, sex, ethnic origin, body mass index, current use of medications for the treatment of migraine attacks, and migraine days, headache days, and acute medication use days per month during the baseline phase), changes in baseline frequency for each endpoint and number of participants experiencing each outcome per randomized group. Only data for the dosages of atogepant that received marketing authorization (10, 30, and 60 mg once daily) [13] were considered in the current systematic review and meta-analysis. Any disagreement was resolved by discussion with a third review author (FB). The risk of bias of the included studies was assessed following the recommendations of the Cochrane Collaboration [15]. Two review authors (SL and FB) used the GRADE approach to judge the certainty of evidence for outcomes based on five criteria (risk of bias, inconsistency, indirectness, imprecision, and publication bias) [16]. If we had serious concerns regarding one of the five criteria, we downgraded the evidence from “high quality” by one level; if we had very serious concerns, we downgraded the evidence by two levels. We resolved any discrepancies through discussion and reported our rationale for downgrading evidence in GRADE table footnotes.
Statistical Analysis
Heterogeneity among the trials was assessed by the chi-squared test and the I2 statistics for heterogeneity. Provided no significant heterogeneity was present (p > 0.05), results were synthesized using a fixed-effect model [15, 17]; if the p value was 0.05 or less, heterogeneity determined the choice of a fixed- or random-effects model for I2 < 40% or ≥ 40%, respectively [18,19,20,21,22]. The mean difference (MD) and risk ratio (RR) with their 95% confidence intervals (CIs) were the measures of association between treatment and continuous/dichotomous outcomes. All efficacy analyses used the modified intention-to-treat population, defined as all randomly assigned participants who received at least one dose of study treatment, had an evaluable baseline period of electronic-diary data, and had at least one evaluable post-baseline 4-week period of electronic-diary data during the double-blind treatment period. Safety and tolerability analyses were done on all randomly assigned participants who received at least one dose of study medication. Reported probability values were two-sided, with significance set at p < 0.05. Data analysis was performed using STATA/IC 13.1 statistical package (StataCorp LP, College Station, TX, USA).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Search Results
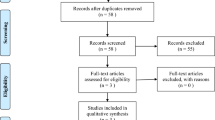
One hundred and one records were identified by database and trial registers searching. Three randomized controlled trials were retrieved for detailed assessment, one of which was still recruiting (ClinicalTrials.gov number, NCT04740827; ELEVATE trial). Accordingly, two studies [23, 24] were considered in the review and included in the meta-analysis (Fig. 1).
Characteristics and Risk of Bias of Included Studies
Both the included studies were multicenter, randomized, double-blind, placebo-controlled trials; one was a phase IIb/III [23] and one a phase III trial, namely the ADVANCE trial [24]. The main characteristics of the studies are summarized in Table 1.
A total of 408 participants were randomized to placebo, 314 to atogepant 10 mg, 411 to atogepant 30 mg, and 417 to atogepant 60 mg once daily. In the study by Goadsby et al. [23], 177 patients were also assigned to atogepant at the dosages of 30 and 60 mg twice daily (data not shown).
The mean age of the patients was 41.0 years and 87.7% were women; white participants were 79.7% of the whole population. The monthly migraine days (mean 7.4), headache days (mean 9.2), and acute medication use days (mean 6.6) reported at baseline were similar across all the studies. Details of the participants are shown in Table 2.
All trials used adequate methods of sequence generation and allocation concealment. We rated all included trials at low risk of performance and detection bias as both atogepant and placebo were provided in identical blister cards, and the patients, site personnel, and trial-sponsor personnel were all masked to the trial-group assignments. The risks of attrition and selective reporting bias were judged low since participants lost to follow-up and withdrawals were documented, and there was no suspicion of selective outcome reporting. Both trials were sponsored by the manufacturer of atogepant; the role of the funding source in the design and conduct of the study, as well as data collection, data management, data analysis, interpretation of findings, and manuscript preparation with support from professional medical writers was disclosed in any trial publication.
Efficacy Outcomes
The reduction in the mean number of migraine days from baseline across the 12-week treatment period was significantly greater among patients treated with atogepant at either the daily dose of 10 mg (MD − 1.16, 95% CI − 1.60 to − 0.73, p < 0.001), 30 mg (MD − 1.15, 95% CI − 1.54 to − 0.76, p < 0.001), or 60 mg (MD − 1.20, 95% CI − 2.18 to − 0.22, p = 0.016) than with placebo (Fig. 2). In comparison to placebo, atogepant at the daily dosage of 10, 30, and 60 mg was associated with a significantly greater reduction in baseline mean number of headache days per month across the 12-week treatment period (atogepant 10 mg: MD − 1.40, 95% CI − 1.88 to − 0.92, p = 0.002; atogepant 30 mg: MD − 1.44, 95% CI − 1.90 to − 0.98, p < 0.001; atogepant 60 mg: MD − 1.48, 95% CI − 1.95 to − 1.02, p < 0.001) (Fig. 3). The reduction from baseline in the mean number of days of use of medication for the treatment of migraine attacks across the 12-week treatment period was significantly greater among patients treated with atogepant at the dose of 10 mg/day (MD − 1.30, 95% CI − 1.74 to − 0.86, p < 0.001), 30 mg/day (MD − 1.40, 95% CI − 1.79 to − 1.01, p < 0.001), and 60 mg/day (MD − 1.30, 95% CI − 1.69 to − 0.91, p < 0.001) than with placebo (Fig. 4).
In comparison to placebo-treated patients, the participants randomized to atogepant were more likely to have at least a 50% reduction in their baseline monthly migraine days at the 10 mg (RR 1.70, 95% CI 1.43–2.03, p < 0.001) (chi squared = 2.90, df = 1, p = 0.089; I2 = 65.5%), 30 mg (RR 1.63, 95% CI 1.07–2.49, p = 0.024) (chi squared = 6.77, df = 1, p = 0.009; I2 = 85.2%), and 60 mg (RR 1.64, 95% CI 1.02–2.66, p = 0.044) (chi squared = 8.71, df = 1, p = 0.003; I2 = 88.5%) daily doses.
The certainty in the evidence for efficacy outcomes was judged to be high (Table e-1 in the Supplementary Material).
Tolerability and Safety Outcomes
Across the trials, AEs were reported by 56.3% and 53.4% of patients treated with atogepant and placebo, respectively; the overall RR to develop any AE during atogepant treatment was 1.07 (95% CI 0.81–1.41, p = 0.630) (chi squared = 6.96, df = 1, p = 0.008; I2 = 85.6%) (Table 3). The certainty in the evidence for this outcome was judged to be moderate; in the GRADE certainty assessment we downgraded once for imprecision as the effect estimate has a wide confidence interval (Table e-1 in the Supplementary Material).
SAEs occurred in 0.6% of the patients randomized to atogepant and 1.0% of those treated with placebo (RR 0.66, 95% CI 0.20–2.20, p = 0.496) (chi squared = 0.75, df = 1, p = 0.385; I2 = 0.0%). Treatment was discontinued because of AEs by 3.5% and 2.7% of patients in the atogepant and placebo groups, respectively; the corresponding RR was 1.32 (95% CI 0.69–2.55, p = 0.402) (chi squared = 0.54, df = 1, p = 0.464; I2 = 0.0%) (Table 3). The certainty in the evidence for these two tolerability/safety outcomes was judged to be low; in the GRADE certainty assessment we downgraded twice for imprecision, as the effect estimate has a wide confidence interval, and because of the very few events that occurred in the groups (Table e-1 in the Supplementary Material).
The incidence rates of the most common AEs in the atogepant- versus placebo-treated participants were as follows: constipation 6.1% versus 1.2%, nasopharyngitis 4.2% versus 2.9%, nausea 6.6% versus 3.2%, upper respiratory tract infection 5.3% versus 6.1%, and urinary tract infection 3.4% versus 2.9%. Nausea was overall more common in atogepant- than placebo-treated patients (RR 2.13, 95% CI 1.19–3.80; p = 0.010) (Table 3). Data on the occurrence of AEs and treatment withdrawal per atogepant doses are summarized in Table 4. Constipation was more commonly observed in patients treated with atogepant at 30 mg/day than placebo (RR 5.19, 95% CI 2.00–13.46; p = 0.001). Treatment with atogepant at the daily dose of 60 mg was associated with a higher risk of constipation (RR 4.92, 95% CI 1.89–12.79; p = 0.001) and nausea (RR 2.73, 95% CI 1.47–5.06; p = 0.001) than placebo.
Discussion
The treatment with orally administered atogepant at the daily doses of 10, 30, and 60 mg resulted in a significantly greater reduction in the number of migraine days and the number of headache days than placebo in adult patients with episodic migraine. Notably, the treatment with atogepant at any dose was associated with a greater rate of participants with a reduction of at least 50% from baseline in the mean number of migraine days per month in comparison to placebo. The effect of atogepant was further substantiated by the significant decrease in the number of days of medication use for the treatment of migraine attacks observed in each atogepant dose group compared with the placebo arm.
A post hoc analysis of the ADVANCE trial data has importantly shown that the benefit of atogepant was already apparent as early as on day 1 of treatment when 10.8–14.1% of participants across the atogepant groups reported migraine versus 25.2% of participants in the placebo group, as well as in each of the first 4 weeks of treatment [25]. The early onset of a sustained therapeutic activity represents an important advantage of atogepant in comparison to many commonly prescribed preventive treatments such as β-blockers, tricyclic antidepressants, and antiseizure medications, which require titration schedules to minimize the risk of side effects and can still have delayed efficacy once the proper maintenance dose is attained [26]. Of note, the rapid onset of action has been already demonstrated with the use of CGRP-targeted monoclonal antibodies as a preventive treatment of migraine in patients with episodic migraine [27,28,29,30]. Although more studies are needed, the currently available evidence suggests how the rapid efficacy may be a characteristic of the drugs targeting the CGRP pathway, regardless of the administration route [25].
The preventive treatment of migraine ultimately has the aim of reducing the disease-related disability. Of note, patient-reported outcomes can provide informative insights on the actual meaningfulness of the treatment effects. In the ADVANCE trial, all three doses of atogepant were associated with a significant improvement in health-related quality of life impairments attributed to migraine, and atogepant at the doses of 30 and 60 mg was associated with a reduction of the detrimental consequences of migraine on daily activities and physical performance, as measured by validated scales [24]. These findings further corroborate the favorable influence that atogepant treatment may have on migraine burden.
Atogepant was generally well tolerated when used in adults with episodic migraine at the dosages of 10, 30, and 60 mg once daily, as shown by the comparable incidence of AEs between the active and placebo arms. The rate of treatment withdrawal among patients treated with atogepant was 3.5% and did not statistically differ from the rate observed among placebo recipients. This risk of treatment discontinuation looks lower in comparison to other oral treatments for migraine prevention [31,32,33] and similar to the risk observed with monoclonal antibodies against CGRP ligand or target [34]. The specificity of action of the agents targeting the CGRP pathways may explain the overall better tolerability profile of this drug class [35].
Constipation and nausea were among the most common AEs, and they were associated with atogepant treatment likely with a dose-dependent relationship. CGRP is widely expressed also in the enteric nervous system and, likewise, the CGRP receptors are present in the gastrointestinal system [36]. Activation of the CGRP receptor induces relaxation of smooth muscle cells and CGRP contributes to maintaining normal intestinal motility [37]. Interestingly, a relationship between blockade of CGRP receptor and reduced gastrointestinal motility was previously reported, and it has been hypothesized that constipation may occur more likely through the antagonism of CGRP receptor than with antibodies neutralizing CGRP [38,39,40]. Indeed, the blockade of the CGRP receptor leaves CGRP free in the system that binds with high affinity to the amylin 1 receptor [41], whose stimulation can inhibit gastric emptying and contribute to constipation. Although no serious cases of constipation occurred during the clinical trials, monitoring and evaluation of atogepant for this AE in clinical practice will be appropriate.
The incidence of SAEs associated with atogepant treatment did not differ with placebo, and no major safety concerns emerged. Although hepatotoxic effects were reported for the first generation of gepants [42], they do not seem to be a class effect. The rate of transaminase elevations over three times the upper limit of normal was similar between patients treated with atogepant and those treated with placebo [13, 23, 24]. There were cases temporally associated with the drug in atogepant recipients, but they were without symptoms and resolved within 8 weeks of treatment discontinuation; no patients had a severe liver injury or jaundice [13]. Of note, the administration of a supratherapeutic dose (170 mg) of atogepant once daily for 28 days was not associated with clinically meaningful alanine aminotransferase elevations in healthy adults [43].
The CGRP is a highly potent vasodilator peptide. The exact role played by the canonical CGRP receptor and the contribution of the CGRP pathway in comparison with other redundant compensatory vasodilator mechanisms are, however, not clear [44]. Atogepant inhibited CGRP-dependent vasodilatory responses in human coronary, cerebral, and middle meningeal arteries in vitro, while it was devoid of vasoconstrictive properties in coronary arteries [45]. In the randomized, double-blind trials, there was no signal of the occurrence of cardiovascular events associated with atogepant [23, 24]. Similarly, clinical studies with CGRP receptor antagonists other than atogepant did not affect cerebral and systemic hemodynamics or vasodilation [46, 47]. Additional studies specifically designed to identify any interference of atogepant with the fundamental homeostatic controls of the vascular tone both at peripheral and cerebral levels will be a useful complement to the currently available information to confirm the cardiovascular safety of this drug.
This systematic review with meta-analysis represents a comprehensive qualitative and quantitative synthesis of the currently available randomized, placebo-controlled, clinical trials of atogepant for the preventive treatment of episodic migraine in adults. This study builds upon the evidence that has focused on atogepant since the availability of the trials’ results and has summarized the milestones in its development from the discovery to place in therapy [48,49,50,51,52]. Of note, results of the analyses of efficacy, tolerability, and safety outcomes were given according to the recommended dosages to better characterize the clinical profile of the drug and provide information that may be useful to physicians in their practice. Nevertheless, the review inherits the limits of the included studies. The question remains about the generalizability of regulatory studies to clinical practice, and real-world evidence will be necessary to evaluate the external validity of clinical trial data. Most of the patients were women and of white ethnic origin, and there were few participants aged 65 years and older. Although no significant effects of sex, race, and age emerged on the pharmacokinetics of atogepant [13], additional evidence is warranted to extend the efficacy and tolerability findings to broader populations. The mean body mass index of the patients, who were all recruited in the USA, was relatively high compared with the body mass index on other continents. Considering the possibility of accumulation in fat tissue, additional research in patients with a lower body mass index will be useful to evaluate if body weight has potential implications for the dosage [53]. The trial excluded patients who had responded inadequately to more than three [23] or four [24] preventive treatments; a multinational phase III trial to assess the safety, tolerability, and efficacy of atogepant 60 mg once daily compared with placebo as a preventive treatment for episodic migraine in adults who previously failed two to four classes of oral prophylactic treatments is recruiting participants (NCT04740827; ELEVATE trial). Participants with 15 or more headache days per month were excluded, and the results cannot be generalized to patients with chronic migraine. The efficacy, tolerability, and safety of atogepant in patients with chronic migraine are now being studied in a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study (NCT03855137; PROGRESS trial). The 12-week treatment duration is not adequate to assess the long-term efficacy and side effects of atogepant. In this regard, atogepant 60 mg administered once daily to patients with episodic migraine over a 52-week open-label, multicenter, phase III extension study (NCT03700320) was efficacious to reduce monthly migraine days, was associated with improvements in function and health-related quality of life, and rates of response increased throughout the course of the trial; further, the treatment was generally well tolerated with no new safety concerns identified [54,55,56]. A number of other studies are ongoing to evaluate the long-term safety and tolerability of atogepant in adult patients with episodic (NCT03939312) and episodic or chronic (NCT04686136 and NCT04437433) migraine. Finally, only two trials, funded by the pharmaceutical company manufacturing atogepant, met the eligibility criteria, and were included in this review. In this regard, the meta-analytic analysis of pooled data allowed us to give a more precise estimate of the effects of the treatment with atogepant for both the efficacy and tolerability outcomes and therefore provided a better characterization of the drug profile in comparison to the single trials.
Conclusion
Drugs targeting the CGRP pathway have been developed in recent years and represent the dawn of a new era in the management of migraine. Atogepant is the second oral gepant to be approved for the preventive treatment of episodic migraine and it is the first and only oral CGRP receptor antagonist developed specifically for migraine prevention [57]. Atogepant comes on top of monoclonal antibodies directed at the CGRP or CGRP receptor, which are also used prophylactically.
CGRP monoclonal antibodies are large molecules that require administration by intravenous or subcutaneous injection and have long half-lives that make monthly or quarterly injections feasible [53]. Compared with monoclonal antibodies, atogepant is a smaller molecule that can be administered orally and has shorter absorption and elimination phases that make daily dosing necessary [53]. Differences in pharmacokinetics and dosing schedules of the medications alongside individual preferences of patients may guide the choice in clinical practice. The variability of available preventive drugs may offer the opportunity to increasingly tailor the treatment and improve the quality of life of patients with migraine.
References
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84:422–35.
Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16:31–8.
Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058–77.
Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain. 2012;13:361–78.
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61.
Lassen LH, Jacobsen VB, Haderslev PA, et al. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain. 2008;9:151–7.
Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86:133–8.
Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81:1191–6.
Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–66.
Cady R, Turner I, Dexter K, Beach ME, Cady R, Durham P. An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache. 2014;54:269–77.
Qulipta-FDA. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf. Accessed Dec 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Higgins JPT and Green S, editors. The Cochrane Collaboration, 2011. http://handbook-5-1.cochrane.org/. Accessed Dec 2021.
GRADE Handbook-Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach [updated October 2013]. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed Jan 2022.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Lattanzi S, Trinka E, Zaccara G, et al. Adjunctive cenobamate for focal-onset seizures in adults: a systematic review and meta-analysis. CNS Drugs. 2020;34:1105–20.
Lattanzi S, Brigo F, Trinka E, et al. Adjunctive cannabidiol in patients with Dravet syndrome: a systematic review and meta-analysis of efficacy and safety. CNS Drugs. 2020;34:229–41.
Lattanzi S, Trinka E, Striano P, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. 2020;61:1090–8.
Lattanzi S, Brigo F, Cagnetti C, Di Napoli M, Silvestrini M. Patent foramen ovale and cryptogenic stroke or transient ischemic attack: to close or not to close? A systematic review and meta-analysis. Cerebrovasc Dis. 2018;45:193–203.
Lattanzi S, Grillo E, Brigo F, Silvestrini M. Efficacy and safety of perampanel in Parkinson’s disease. A systematic review with meta-analysis. J Neurol. 2018;265:733–40.
Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727–37.
Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695–706.
Schwedt TJ, Lipton RB, Ailani J, et al. Time course of efficacy of atogepant for the preventive treatment of migraine: results from the randomized, double-blind ADVANCE trial. Cephalalgia. 2022;42:3–11.
Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19:92.
Vernieri F, Altamura C, Brunelli N, et al. Rapid response to galcanezumab and predictive factors in chronic migraine patients: a 3-month observational, longitudinal, cohort, multicentre, Italian real-life study. Eur J Neurol. 2021. https://doi.org/10.1111/ene.15197.
Winner PK, Spierings ELH, Yeung PP, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache. 2019;59:1743–52.
Dodick DW, Gottschalk C, Cady R, et al. Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on day 1 after dosing. Headache. 2020;60:2220–31.
Lattanzi S, Brigo F, Trinka E, et al. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs. 2019;79:417–31.
Silberstein SD, Neto W, Schmitt J, Jacobs D, MIGR-001 Study Group. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61:490–5.
Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291:965–73.
Vécsei L, Majláth Z, Szok D, Csáti A, Tajti J. Drug safety and tolerability in prophylactic migraine treatment. Expert Opin Drug Saf. 2015;14:667–81.
Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol. 2012;166:66–78.
Diener HC, Förderreuther S, Gaul C, et al. Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: addition to the S1 guideline: therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society. Neurol Res Pract. 2020;2:11.
Jansen I, Uddman R, Hocherman M, et al. Localization and effects of neuropeptide Y, vasoactive intestinal polypeptide, substance P, and calcitonin gene-related peptide in human temporal arteries. Ann Neurol. 1986;20:496–501.
Mulderry PK, Ghatei MA, Spokes RA, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205.
Falkenberg K, Bjerg HR, Olesen J. Two-hour CGRP infusion causes gastrointestinal hyperactivity: possible relevance for CGRP antibody treatment. Headache. 2020;60:929–37.
Barbanti P, Aurilia C, Egeo G, et al. Erenumab in the prevention of high-frequency episodic and chronic migraine: erenumab in real life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache. 2021;61:363–72.
Barbanti P, Aurilia C, Cevoli S, et al. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: results of the EARLY 2 study. Headache. 2021;61:1351–63.
Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR review 25. Br J Pharmacol. 2018;175:3–17.
Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs. 2019;28:555–67.
Min KC, Kraft WK, Bondiskey P, et al. Atogepant is not associated with clinically meaningful alanine aminotransferase elevations in healthy adults. Clin Transl Sci. 2021;14:599–605.
Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–142.
Rubio-Beltran E, Chan KY, Danser AJ, et al. Characterisation of the calcitonin gene-related peptide receptor antagonists ubrogepant and atogepant in human isolated coronary, cerebral and middle meningeal arteries. Cephalalgia. 2020;40:357–66.
Petersen KA, Birk S, Lassen LH, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–47.
Van der Schueren BJ, Blanchard R, Murphy MG, et al. The potent calcitonin gene-related peptide receptor antagonist, telcagepant, does not affect nitroglycerin-induced vasodilation in healthy men. Br J Clin Pharmacol. 2011;71:708–17.
Deeks ED. Atogepant: first approval. Drugs. 2022;82:65–70.
Tao X, Yan Z, Meng J, et al. The efficacy and safety of atogepant for the prophylactic treatment of migraine: evidence from randomized controlled trials. J Headache Pain. 2022;23:19.
Singh A, Balasundaram MK. Atogepant for migraine prevention: a systematic review of efficacy and safety. Clin Drug Investig. 2022;42:301–8.
Rustichelli C, Avallone R, Ferrari A. Atogepant: an emerging treatment for migraine. Expert Opin Pharmacother. 2022;23:653–62.
Cohen F, Yuan H. Role of atogepant in the treatment of episodic migraines: clinical perspectives and considerations. Ther Clin Risk Manag. 2022;18:447–56.
Al-Hassany L, Van Den Brink AM. Targeting CGRP in migraine: a matter of choice and dose. Lancet Neurol. 2020;19:712–3.
Ashina M, Tepper SJ, Reuter U, et al. Atogepant 60 mg once-daily shows efficacy for the preventive treatment of migraine: results from a 52-week open-label extension trial [abstract no. P-133]. Headache. 2021;61(Suppl 1). https://doi.org/10.1111/head.14130.
Lipton RB, Halker Singh RB, Mechtler LL, et al. Daily dosing of atogepant for preventive treatment of migraine improved patient reported outcomes measures of migraine-specific quality of life, activity impairment in migraine-diary, and headache-impact test in a 52-week trial [abstract no. P-203]. Headache. 2021;61(Suppl 1):119–20.
Ashina M, Tepper S, Reuter U, et al. Long-term safety and tolerability of atogepant 60 mg following once daily dosing over 1 year for the preventive treatment of migraine [abstract]. Neurology. 2021;96(15 Suppl 1). https://n.neurology.org/content/96/15_Supplement/2664.full. Accessed Dec 2021.
Martelletti P, Cipolla F, Capi M, Curto M, Lionetto L. Atogepant. Calcitonin gene-related peptide (CGRP) receptor antagonist, preventive treatment of migraine. Drugs Future. 2020;45:285.
Acknowledgements
Funding
No funding has been received for the conduct of this study or publication of this article.
Author Contributions
Simona Lattanzi designed and conceptualized the study, carried out the data analyses, and drafted the manuscript. Cinzia Del Giovane carried out the data analyses. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript for submission and agree to be accountable for all aspects of the work.
Disclosures
Simona Lattanzi has received speaker's or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, and UCB Pharma and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, and GW Pharmaceuticals. Eugen Trinka received speaker’s honoraria from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, Sunovion Pharmaceuticals Inc., LivaNova and Novartis; consultancy funds from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, GW Pharmaceuticals, Sunovion Pharmaceuticals Inc., and Novartis; directorship funds from Neuroconsult GmbH. E. Trinka’s Institution received grants from Biogen, Red Bull, Merck, UCB Pharma, European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bundesministerium für Wissenschaft und Forschung. Claudia Altamura has received grants and honoraria from Novartis and Eli Lilly. Fabrizio Vernieri has received travel grants or honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Amgen, Angelini, Eli Lilly, Lundbeck, Novartis, and Teva. Cinzia Del Giovane, Mauro Silvestrini, and Francesco Brigo have no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lattanzi, S., Trinka, E., Altamura, C. et al. Atogepant for the Prevention of Episodic Migraine in Adults: A Systematic Review and Meta-Analysis of Efficacy and Safety. Neurol Ther 11, 1235–1252 (2022). https://doi.org/10.1007/s40120-022-00370-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00370-8