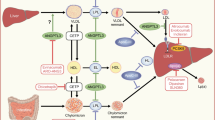
Abstract
Introduction
This study characterizes patients receiving evolocumab in clinical practice and assesses treatment effectiveness, safety and persistence outcomes across five countries.
Methods
This retrospective and prospective observational study enrolled patients initiated on evolocumab during August 2017 to July 2019 at 49 sites across Canada, Mexico, Colombia, Saudi Arabia and Kuwait. Medical records data were extracted within 6 months prior to (baseline) and every 3 months for 12 months post evolocumab initiation and reported as available.
Results
A total of 578 patients were enrolled (40.1% female, median age 60 [interquartile range (IQR) 51–68] years); 83.7% had atherosclerotic cardiovascular disease and/or familial hypercholesterolemia. Median low-density lipoprotein cholesterol (LDL-C) at baseline was 3.4 (IQR 2.7–4.2) mmol/L (131.5 [IQR 104.4–162.4] mg/dL), with 75.6% of patients receiving a statin (59.2% high intensity). Compared to baseline, the median lowest LDL-C was reduced by 70.2% and remained stable over 12 months of treatment. Guideline-recommended LDL-C thresholds < 1.8, < 1.4 and < 1.0 mmol/L (< 70, < 55 and < 40 mg/dL) were achieved by 75.3%, 63.6% and 47.4% of patients. LDL-C outcomes were consistent across high- and very high-risk patients. Background lipid-lowering therapy remained relatively stable. No serious treatment-emergent adverse events were reported, and persistence to evolocumab was 90.2% at 12 months.
Conclusion
These findings provide real-world evidence that evolocumab use is in accordance with its international guideline-recommended place in dyslipidemia therapy, as well as confirmation of its effectiveness and safety in a heterogeneous population. Evolocumab can address a healthcare gap in the management of dyslipidemia by increasing the proportion of patients achieving LDL-C goals recommended to lower cardiovascular risk.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The ZERBINI Study is the first intercontinental real-world evaluation of evolocumab use, effect on low-density lipoprotein cholesterol (LDL-C) reduction, safety and persistence to therapy over time, conducted in patients with atherosclerotic cardiovascular disease (ASCVD) and/or familial hypercholesterolemia (FH) in countries in North America, South America and the Middle East. |
Compared to baseline, median LDL-C was reduced by 70.2% and remained stable over 12 months of treatment with evolocumab. |
Most patients achieved below international guideline-recommended LDL-C thresholds and outcomes remained consistent across high-risk and very high-risk patients. |
No serious treatment-emergent adverse events were reported and persistence to evolocumab was 90.2% at 12 months. |
The ZERBINI Study provides real-world confirmation of the effectiveness and safety of evolocumab in a heterogeneous population, thereby supporting a role for evolocumab to address a healthcare gap in the management of dyslipidemia by increasing the proportion of patients achieving LDL-C goals recommended to lower cardiovascular risk. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24174990.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of mortality responsible for approximately one-third of all deaths worldwide, with most (85%) attributed to myocardial infarction (MI) and stroke [1]. Lowering low-density lipoprotein cholesterol (LDL-C) levels is a well-established approach to primary and secondary prevention of ASCVD [2]. Indeed, robust randomized clinical data on the use of several available pharmacologic lipid-lowering therapies (LLT) consistently support a causal and cumulative reduction in the risk of ASCVD, estimated up to 20–25% for every 1 mmol/L (40 mg/dL) reduction in LDL-C over 5 years, regardless of the type of LLT used [3].
International guidelines for dyslipidemia management from the American College of Cardiology and American Heart Association (ACC/AHA) in 2018, European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) in 2019, and Canadian Cardiovascular Society (CCS) in 2021 recommend LLT to achieve target LDL-C thresholds in patients at high and very high cardiovascular (CV) risk, with some nuances between guidelines in the criteria for these categories [4,5,6]. For primary prevention in patients with severe hypercholesterolemia without ASCVD, such as with familial hypercholesterolemia (FH), the CCS and ACC/AHA recommend respective LDL-C thresholds of < 2.5 mmol/L (< 96.7 mg/dL) and < 2.6 mmol/L (< 100.5 mg/dL), and/or a ≥ 50% reduction from baseline, whereas the ESC/EAS recommend < 1.8 mmol/L (< 70 mg/dL) and a ≥ 50% reduction [4,5,6]. For secondary prevention in patients with established ASCVD with/without FH or other comorbid risk factors, the CCS and ACC/AHA recommend a LDL-C of < 1.8 mmol/L, whereas the ESC/EAS now recommend < 1.4 mmol/L (< 55 mg/dL), and both the ACC/AHA and ESC/EAS recommend a concomitant ≥ 50% reduction [4,5,6]. Lastly, specifically for patients with ASCVD who experience a second vascular event within 2 years, the ESC/EAS now recommend a LDL-C of < 1.0 mmol/L (< 40 mg/dL) [5].
International guidelines agree high-intensity statins are the recommended first-line LLT for CV risk reduction owing to their low cost and proven efficacy [4,5,6], with an expected LDL-C reduction of approximately 50% [7]. However, real-world evidence consistently reveals that a substantial proportion of patients at high risk of ASCVD, or with established ASCVD, do not achieve guideline-recommended LDL-C thresholds despite treatment [8,9,10]. In patients where maximally tolerated statin therapy alone is not sufficient to reach guideline-recommended LDL-C thresholds, LLT intensification via the addition of ezetimibe is recommended for an expected incremental 20% reduction in LDL-C [4,5,6, 11, 12]. In addition, or as an alternative, to ezetimibe in patients still above recommended LDL-C levels, a proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitor (monoclonal antibody) is recommended [4,5,6]. Inhibition of PCSK9 has been shown to prevent PCSK9-mediated LDL receptor degradation and thereby lower serum LDL-C [13]. Still, in the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD-2) registry of 2651 US patients with ASCVD and a LDL-C ≥ 1.8 mmol/L, only 14.4% had LLT intensification after 2 years, with only 2.2% initiated on a PCSK9 inhibitor [14]. As an adjunct to statin therapy in patients with FH or established ASCVD, a systematic review and network meta‐analysis of randomized controlled trials showed that the PCSK9 inhibitor evolocumab may reduce LDL-C up to 74.1% in combination with statin therapy [15]. These data are reinforced by a growing body of real-world evidence of evolocumab effectiveness within specific countries or continents [14, 16,17,18,19]. This includes the CHaractEristics of HYperlipidaeMic PAtieNts at Initiation of Evolocumab and Treatment PatternS (HEYMANS) study, which characterized real-world evolocumab use across 12 European countries; however, there remains an unmet need for a systematic analysis of real-world and routine evolocumab use and outcomes across diverse populations, healthcare settings and reimbursement landscapes, and according to current international lipid guidelines.
The multiZonal obsERvational study conducted By clinIcal practitioners on Repatha® (Evolocumab) use iN subjects with hyperlipIdemia (ZERBINI) is the first intercontinental real-world evaluation of evolocumab use in countries in North America, South America and the Middle East. The primary objective was to characterize the patient profile receiving evolocumab in each country, including disease status and background LLT usage. Secondary and exploratory objectives were to evaluate the effect of evolocumab on LDL-C reduction and international guideline-recommended LDL-C achievement according to CV risk status, as well as safety and persistence to therapy over time.
Methods
Study Design, Setting and Participants
This was a retrospective and prospective observational study of patients ≥ 18 years of age from 49 sites across five countries (Canada, Mexico, Colombia, Saudi Arabia and Kuwait; investigators listed in Table S1), wherein patients were initiated on evolocumab as part of routine clinical care (based on local reimbursement criteria, Table S2). The selected countries were included to characterize evolocumab use across diverse populations, healthcare settings and reimbursement landscapes to expand on what has been previously reported. Additionally, the selected sites were among first potential prescribers of evolocumab and had experience with this new molecule in the clinical setting. Patients were eligible if they (i) were male or female ≥ 18 years of age at the time of signing the informed consent form, (ii) initiated evolocumab at a physician’s discretion between August 1, 2017 and July 9, 2019, (iii) received at least one dose of evolocumab, and (iv) had ≤ 6 months exposure to evolocumab prior to study enrollment. Patients were excluded if they had used a PCSK9 inhibitor within 6 months prior to evolocumab initiation. Six hundred and ten patients were assessed for eligibility and 578 were included in the final cohort (Fig. S1). Thirty-two patients were excluded from the full analysis set because of failure to meet the inclusion criteria. Examples include inability to access commercial drug, or the primary investigator could not confirm if the patient received at least one dose of evolocumab before into the study. Data were collected from patient medical records up to 6 months prior to evolocumab initiation (baseline) and every 3 months for 12 months post initiation, regardless of continuation or discontinuation of evolocumab, with data collection ending on July 6, 2020 (Fig. S2). The study was conducted in accordance with the Declaration of Helsinki and the study protocol was reviewed and approved by each site’s institutional review board/institutional ethics committee. Participants were required to sign an informed consent form for inclusion in the study.
Variables of Interest, Data Collection and Analyses
Available data were collected from patient medical records using a case report form (CRF). Given the possibility of differences in data capture between sites, training was provided using study-specific eCRFs on methods for data extraction from the subject’s medical record. Descriptive statistics were used to summarize study outcomes for the subsets of patients with available data for each endpoint (Fig. S1). The RECORD statement was used to guide this report [20].
Patient Demographic and Clinical Characteristics
Variables of interest were chosen to reflect parameters used in routine clinical management of ASCVD and are expected to provide data which are of interest to physicians and health authorities and relevant in the context of current clinical practice. These variables of interest included the following patient demographic and clinical characteristics at baseline upon evolocumab initiation: age; sex; race; smoking status; ASCVD status, including number of ASCVD conditions; FH status, including subtype (heterozygous or homozygous); diabetes status; and LDL-C concentration, with the last measure within 6 months prior to evolocumab initiation regarded as the baseline value. Notably, patients with any of the following conditions were classified as having ASCVD based on the definition included in current international guidelines [4,5,6], as previously described [18]: angina; abdominal aortic aneurysm; carotid or coronary artery disease (CAD); coronary revascularization procedures, including coronary artery bypass grafting; percutaneous transluminal coronary angioplasty; peripheral artery disease; intermittent claudication; MI; stroke; and transient ischemic attack. Patients were then stratified by clinically relevant subgroups: ASCVD without FH, ASCVD with FH, FH without ASCVD, and neither FH nor ASCVD, as previously described.
Evolocumab and LLT Usage
Additional variables of interest included evolocumab dosage and background LLT usage (type, dosage) at baseline and changes over the 12-month follow-up period. The statin name and dose were reported by the investigator and statin intensity was grouped into low, moderate and high intensity according to the 2013 ACC/AHA guideline categorizations [7]. Statin intolerance (SI) was determined at investigators’ discretion, with the number of statins patients were intolerant to also reported.
Evolocumab Effectiveness and LDL-C Measurement Characteristics
Evolocumab effectiveness was assessed as the change in LDL-C from baseline as well as achievement of the ACC/AHA, ESC/EAS and CCS LDL-C recommended values of < 1.8, < 1.4 and < 1.0 mmol/L (< 70, < 55 and < 40 mg/dL, respectively, and presented henceforth in mmol/L) during the 12-month follow-up period [4,5,6]. These endpoints were assessed for the full study cohort as well as for patient subgroups of clinical interest based on the evolocumab indication [21] and patients expected to gain the greatest benefit from LLT intensification with a PCSK9 inhibitor, namely those at high (ASCVD without FH, FH without ASCVD) and very high (ASCVD with FH; ≥ 2 ASCVD conditions, with or without FH; ASCVD with diabetes, with or without FH) CV risk according to international guidelines [4,5,6]. The lowest LDL-C measure was used for patients with multiple measures within the presented timeframes, as previously described [19]. To account for the real-world nature of the study design wherein not all patients were guaranteed to be on evolocumab at the time of their LDL-C measurement(s), a sensitivity analysis of LDL-C outcomes using patients’ mean LDL-C over the 12-month follow-up period was conducted in up to 409 patients who remained on evolocumab at the end of the study (‘completers’), to generate an accurate representation of the real-world impact of evolocumab use on dyslipidemia. Ultimately, LDL-C outcomes were consistent between the full cohort and completers (Table S3). Lastly, LDL-C measurement characteristics at baseline and follow-up were also assessed, including the incidence, frequency and time to first and last measurement.
Evolocumab Safety and Persistence
Other variables of interest over the 12-month follow-up period included safety, assessed as the incidence of adverse events (AEs); study participation (completed or discontinued); and persistence to evolocumab. Data on missed evolocumab doses (incidence and number) were captured in the CRF on a weekly basis. Persistence was then calculated as previously described [18], as the proportion of patients remaining on evolocumab for the entire follow-up period without missing doses for ≥ 56 consecutive days, the allowable gap based on the evolocumab dosing instructions [21]. Patients who discontinued study participation for an AE, death, requirement for an alternative therapy, or unknown reasons were captured as non-persistent. Those who discontinued study participation for reasons deemed unrelated to evolocumab (reimbursement, study enrollment deviation, patient request, clinician decision and lost to follow-up) were not included in the persistence calculation (N = 117).
Results
Patient Baseline Demographic and Clinical Characteristics
The cohort consisted of 578 patients prescribed evolocumab as part of routine care. Patient demographics and clinical characteristics at baseline are provided in Table 1. The median (interquartile range [IQR]) age was 60 (51–68) years, 33.4% of patients were < 55 years old, and 40.1% were female. A diversity of races was represented with 62.8% of patients being Middle Eastern, Latino, Asian, Mixed/Biracial, American Indian or Alaska Native, Black or African American, South Asian or of unknown race. The median (IQR) LDL-C concentration at baseline prior to evolocumab initiation was 3.4 (2.7–4.2) mmol/L (131.5 [104.4–162.4] mg/dL) (N = 539), which was relatively consistent across the five countries, with the exception of Kuwait having a lower baseline LDL-C (2.8 [2.3–3.6] mmol/L; 108.3 [88.9–139.2] mg/dL). Median (IQR) baseline LDL-C concentration in women (N = 216) was 3.8 (3.0–4.7) mmol/L (145.5 [116.7–181.2] mg/dL), and in men (N = 323) was 3.2 (2.5–4.0) mmol/L (125.3 [96.7–155.8] mg/dL) (data not shown). Of the full cohort, 62.1% had ASCVD without documented FH, 12.8% had ASCVD with FH, and 8.8% had FH without ASCVD. Ninety-four (16.3%) patients had neither FH nor ASCVD diagnosis. Of note, Canada had proportionately more patients with FH (55.0%) compared to the other countries (≤ 16.1%). The median (IQR) age of patients with ASCVD with or without FH was 62 (53–69) years, and that of patients with FH without ASCVD was 60 (49–66) years (data not shown). Among patients with ASCVD with or without FH, the majority (71.6%) had ≥ 2 conditions, with CAD (68.2%) and MI (46.9%) being the most common conditions. Among the comorbid conditions analyzed, 39.1% of patients had diabetes, with the greatest prevalence occurring in the Middle East (58.6–61.9%). Notably, there was no change in hemoglobin A1C over the study period, in patients with and without diabetes (data not shown).
Background Lipid-Lowering Therapies and Evolocumab Usage Over Study Period
Background LLT usage (type, dosage) and the dosage of evolocumab initiated at baseline are presented in Table 2 and Fig. 1 (breakdown by country in Table S4). Of the 578 patients in this cohort, background statin use was reported in 75.6% at baseline, with 59.2% receiving a high intensity statin. Reported SI varied considerably by country (7.7% in Kuwait to 61.8% in Canada) with most patients (57.8%) deemed intolerant to only one statin. Of note, Mexico had 43.5% of patients with neither FH nor ASCVD but only 40.4% of them had SI, which was consistent with other countries (31.3% in Kuwait to 62.5% in Canada). Further, 35.8% of patients were receiving ezetimibe at baseline, with 29.1% of patients receiving combined statin and ezetimibe therapy. Evolocumab was prescribed at a dose of 140 mg every 2 weeks in most (98.8%) patients (vs. 420 mg every 4 weeks, which is not available in all studied countries) and as monotherapy in 15.4% of patients at baseline.
Background lipid-lowering therapies during study period. *The definition of statin intensity was based on the 2013 American College of Cardiology/American Heart Association Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. The highest intensity was considered if statins with multiple intensities were used at the same time. If there was a change of dose over the reporting interval, the dose closest to the end of the interval was used. The statin intensity was unknown in 2 patients at baseline, and 1 patient throughout months 1–12 of follow-up. LLT lipid-lowering therapy
Lipid-lowering therapy usage over time is presented in Fig. 1 and remained relatively stable over the 12-month follow-up period post evolocumab initiation. Among all patients on a statin at baseline (N = 437), 2.5% had modifications to their statin dosage, including 2.1% who downtitrated and 0.5% who uptitrated statins. Among all patients on a background statin and/or ezetimibe at baseline (N = 476), 6.3% discontinued their background therapies during follow-up. Seven (1.5%) patients on background statin + ezetimibe discontinued ezetimibe during follow-up. Lastly, among all patients on evolocumab monotherapy at baseline (N = 89), 3.6% started additional LLT during follow-up (data not shown).
LDL-C Measurements and Characteristics Over Study Period
The incidence and characteristics of LDL-C measurements over time are presented in Table S5. Thirty-nine (6.7%) patients did not have a LDL-C measure reported within 6 months prior to evolocumab initiation. Most patients (77.0%) had ≥ 1 LDL-C measurement post evolocumab initiation, with a median (IQR) time to first and last test of 87 (46–148) days and 247 (153–315) days, respectively.
Changes in LDL-C Concentrations from Baseline During Follow-up
In the 445 patients with available data, the median (IQR) LDL-C concentration during the 12-month follow-up post evolocumab initiation was 1.0 (0.6–1.8) mmol/L (38.7 [23.2–69.6] mg/dL) (data not shown). Baseline and corresponding follow-up LDL-C concentrations are shown in Fig. 2. In patients with available data at both baseline and over the 12-month follow-up period (N = 417), LDL-C was reduced by a median (IQR) 70.2% (− 51.3% to − 81.5%), which was consistent between men vs. women (− 71.3%, N = 248 vs. − 67.3%, N = 169; data not shown). This was achieved during months 1–6 post evolocumab initiation and maintained during months 7–12 in patients with LDL-C measurements at both timepoints (N = 215) (Fig. S3). Similar LDL-C reductions were achieved by subgroups of clinical interest over the 12-month follow-up period post evolocumab initiation, including those at high CV risk (ASCVD without FH, − 71.0%, N = 247; FH without ASCVD, − 65.8%, N = 43) and very high CV risk (ASCVD with FH, − 70.4%, N = 61; ≥ 2 ASCVD conditions with or without FH, − 71.6%, N = 221; ASCVD with diabetes with or without FH, − 70.3%, N = 120) (Fig. 2). Finally, patients on evolocumab monotherapy achieved a median (IQR) 62.5% (45.5–74.3) LDL-C reduction from baseline (Fig. S4).
Median LDL-C concentrations at baseline and follow-up in the full study cohort* and subgroups of clinical interest. *Data represent patients with a LDL-C measure at baseline (measured within 6 months prior to initiation of evolocumab) and their minimum LDL-C measure during the 12-month study follow-up period; †With or without FH. ASCVD atherosclerotic cardiovascular disease, FH familial hypercholesterolemia, IQR interquartile range, LDL-C low-density lipoprotein cholesterol
Changes in LDL-C from baseline over the 12-month follow-up period in each individual patient with available data at both timepoints (N = 417) are shown in Fig. 3. LDL-C was reduced in 97.4% of patients post evolocumab initiation, with 89.2% achieving a ≥ 30% reduction, 76.0% achieving a ≥ 50% reduction, and 27.6% achieving a ≥ 80% reduction (Table S6). Of 11 patients who had no apparent reduction in LDL-C over 12 months post evolocumab initiation, three had discontinued evolocumab and one had missed two consecutive doses (28 days of no drug). Six of the 11 patients had unknown FH status. Notably, a ≥ 50% LDL-C reduction from baseline was achieved by 75% of patients with FH (± ASCVD) (data not shown), as recommended by international guidelines [4,5,6].
Distribution of LDL-C reductions from baseline during study period in the full study cohort (N = 417). **Data represent patients with a LDL-C measure at baseline (measured within 6 months prior to initiation of evolocumab) and their minimum LDL-C measure during the 12-month study follow-up period. LDL-C low-density lipoprotein cholesterol
Achievement of Guideline-Recommended LDL-C Thresholds During Follow-up
The proportions of patients in the full cohort and subgroups of clinical interest with ≥ 1 LDL-C measure during the 12-month follow-up period post evolocumab initiation who achieved guideline recommended LDL-C thresholds during follow-up are shown in Fig. 4. In the full cohort with available data (N = 445), 75.3%, 63.6% and 47.4% patients achieved guideline-recommended LDL-C values of < 1.8, < 1.4 and < 1.0 mmol/L, respectively [4,5,6]. These proportions were consistent among patients at high and very high CV risk. Namely, 81.2% of patients with ASCVD without FH (N = 260) achieved below the CCS and ACC/AHA-recommended threshold of 1.8 mmol/L [4, 6], and 71.5% achieved below the ESC/EAS-recommended threshold of 1.4 mmol/L [5]. Similar proportions of patients with ≥ 2 ASCVD conditions and ASCVD with diabetes (with or without FH) achieved the same LDL-C thresholds. In patients with FH, 65.2% of those without ASCVD (N = 46) and 69.2% with ASCVD (N = 65) achieved a LDL-C < 1.8 mmol/L, and 54.4% and 56.9% achieved < 1.4 mmol/L, respectively. Notably, approximately half of the full cohort as well as all patient subgroups achieved the ESC/EAS-recommended LDL-C goal of < 1.0 mmol/L for patients with a second vascular event within 2 years, including 56.3% of patients with ≥ 2 ASCVD conditions [5].
Proportion of patients who achieved guideline-recommended LDL-C thresholds at follow-up: full study cohort and subgroups of clinical interest*. *Data represent patients’ minimum LDL-C measure during the 12-month study follow-up period; †With or without FH. ASCVD atherosclerotic cardiovascular disease; FH familial hypercholesterolemia, LDL-C low-density lipoprotein cholesterol
Adverse Events During Follow-up
A summary of AEs is presented in Table 3 and the full list of AEs is presented in Table S7. Most patients (96.7%) did not have any AEs reported and no serious AEs were reported. The most common AEs were balance disorder/dizziness (0.9%), myalgia (0.5%) and headache (0.5%), with 1 (0.2%) puncture site ecchymosis reported. CV-related hospitalizations were reported in 5.0% of patients, although the cause (i.e. CV event or scheduled procedure) is unknown (data not shown). AEs leading to discontinuation of evolocumab (0.9%) were non-serious. Reasons for study discontinuation over the 12-month follow-up period are presented in Table 4. Of the 20.2% of patients who discontinued the study, being lost to follow-up was the most common reason (22.0%).
Evolocumab Persistence During Follow-up
Evolocumab persistence was assessed in the remaining 461 patients and was 90.2%, as presented in Table 5. The most reported reason for non-persistence was ‘missed doses for ≥ 56 consecutive days’ (3.3%). Additionally, persistence was similar between patients on evolocumab monotherapy (89.1%, N = 55) and those on background LLT (90.4%, N = 406).
Discussion
This analysis of the full ZERBINI study population is the first systematic intercontinental evaluation of real-world evolocumab use and outcomes for dyslipidemia management. The current findings from this international study are largely consistent with the approved indications [21], international guideline recommendations [4,5,6] and local reimbursement criteria (Table S2) for evolocumab, in that evolocumab was predominantly used in patients with ASCVD, FH or both (representing 83.7% of patients), with a significant proportion having multiple ASCVD and/or comorbid conditions. Further, 75% of patients had a LDL-C ≥ 2.7 mmol/L (≥ 104.4 mg/dL), and 82.4% were receiving a background statin and/or ezetimibe at the time of evolocumab initiation. The patient profile initiated on evolocumab in North America, South America and the Middle East reported herein and in recent continental sub-analyses [18], Roncancio et al. (submitted for publication), and [22] provides important additional diversified and global perspectives to that observed in the HEYMANS study, the largest real-world European study of evolocumab use to date [16]. Within the 1952 patients across the 12 countries studied in HEYMANS, 97% had ASCVD, FH or both, with 75% having a LDL-C of ≥ 3.17 mmol/L (≥ 122.6 mg/dL), yet only 59% were receiving a background statin and/or ezetimibe at evolocumab initiation [16], perhaps reflecting differences in local dyslipidemia management practices and/or evolocumab reimbursement criteria across countries and continents. Notably, the prevalence of MI/acute coronary syndrome was consistent between the current ZERBINI (46.9%) and HEYMANS (42%) studies [16]. Accordingly, the clinical characteristics of most patients in this study cohort are aligned with the approved indication [21] and guideline recommendations for evolocumab usage [4,5,6], with it being primarily prescribed for patients at high and very high CV risk whose LDL-C remains elevated despite maximally tolerated statin and/or ezetimibe therapy.
Differences in local practice patterns and evolocumab reimbursement criteria (Table S2) may underscore differences in the specific evolocumab patient profile observed in each country herein. For instance, Canada had a higher proportion of patients with FH (55.0%) compared to the other countries (≤ 16.1%) even though evolocumab is publicly reimbursed for FH in all of them, suggesting Canadian patients with FH may be prioritized for evolocumab initiation. Also noteworthy, the prevalence of patients with ≥ 2 ASCVD conditions as defined in the current study was higher in the Middle East (Saudi Arabia, 71.0% and Kuwait, 54.3%) and Colombia (71.1%) compared to North America (≤ 42.0%). In the Middle East in particular, public access to evolocumab for ASCVD is restricted to patients with a LDL-C well above the ESC/EAS-recommended target for LLT intensification with a PCSK9 inhibitor in patients with ASCVD (> 1.4 mmol/L) [5], being > 2.6 mmol/L (> 100 mg/dL) in Saudi Arabia and > 1.8 mmol/L (> 70 mg/dL) in Kuwait. Further, the prevalence of diabetes was higher in the Middle East (≥ 58.6%) than the other countries (≤ 32.4%), resulting in a higher prevalence of diabetes in the full ZERBINI study cohort (39.1%) compared to that in HEYMANS (19%) [16]. This is consistent with estimates showing the Middle East has the highest prevalence of diabetes worldwide [23] and collectively suggests patients at very high CV risk are prioritized for evolocumab use in this region. Finally, reported SI was highest in Canada (61.8%) compared to the other countries studied (7.7–44.4%), yet in line with that reported in the HEYMANS study (60%) [16], which may reflect the requirement to document SI for evolocumab reimbursement in Canada and many European countries, and/or a lack of consensus on the definition and diagnosis of SI across countries.
Upon evolocumab initiation, at least half of all patients in each of the studied countries had LDL-C concentrations at least double those recommended for LLT intensification in international guidelines [4,5,6], yet almost 25% were not receiving recommended first-line statin therapy. While these data are reassuring compared to the 57% of European patients not receiving a background statin at evolocumab initiation in the HEYMANS study [16], it points to significant worldwide care gaps in dyslipidemia management. One contributing factor may be infrequent LDL-C monitoring, despite the ESC/EAS recommendation to measure lipids within 1–3 months following LLT initiation [5]. In the current study, while only 6.7% of patients did not have a LDL-C measurement within 6 months prior to evolocumab initiation, 23.0% did not have a follow-up LDL-C measurement within 12 months post evolocumab initiation. Whether this reflects therapeutic complacency or a “fire and forget” practice pattern is not known, but it highlights the continued need for guidance and implementation of routine LDL-C measurements in vulnerable patients to identify candidates for LLT intensification and optimization. Reassuringly, in patients with ≥ 1 follow-up LDL-C measurement, most (60.9%) had ≥ 2 measurements over 12 months and half had their first measurement within 3 months, which may reflect local reimbursement requirements and/or suggest lipid monitoring is prompt and thorough when prioritized in clinical practice.
The 70.2% reduction in LDL-C observed over 12 months post evolocumab initiation in this real-world analysis validates the 65.0–67.6% reduction averaged over the dosing interval as reported in randomized clinical trials [24]. In the current study, a clinically significant reduction in LDL-C was observed during months 1–6 post evolocumab initiation, and maintained during months 7–12. Likewise, in the HEYMANS study, LDL-C was reduced by 58.1% within 3 months post evolocumab initiation and maintained for 2 years [16]. A similar LDL-C reduction of 58.4% was maintained for a median of 7 years post evolocumab initiation in 3355 European and US patients in the Open Label Extension of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk trial (FOURIER-OLE) [25]. Interestingly, patients originally randomized to receive evolocumab during the parent FOURIER trial had a 15–20% lower risk of major adverse CV events and 23% lower risk of CV death compared to those originally randomized to placebo, despite both groups receiving evolocumab during the OLE [25]. Hence, the current findings reinforce the real-world effectiveness of evolocumab for prompt, significant and sustained LDL-C clearance in a heterogenous patient population. Indeed, LDL-C was reduced to some extent in almost all (97.4%) patients and by ≥ 30% in 89.2% and by ≥ 50% in 76.0% of patients over 12 months. These data reassure clinically significant LDL-C reductions in the real-world setting consistent with that in the FOURIER trial wherein LDL-C was reduced by ≥ 50% in 94.7% of patients within the same duration [26]. The biological basis for suboptimal response to PCSK9 inhibition in the remaining 2.6% of patients in the current real-world study warrants further investigation but may be explained by factors relevant to routine clinical practice, including a lack of adherence to LLT, dietary or lifestyle changes, and/or human error or technical faults during sample handling or analyses, among others.
LDL-C reductions post evolocumab initiation were consistent across patients at high and very high CV risk. Particularly among patients with ASCVD without FH in the current study, 81.2% achieved a LDL-C < 1.8 mmol/L. These real-world data are comparable to the FOURIER trial wherein 87% of patients achieved a LDL-C < 1.8 mmol/L versus only 18% of patients in the placebo arm on statin monotherapy [27], reinforcing the clinical benefit of LLT intensification with evolocumab. Among patients with FH in the current study, 67.6% achieved a LDL-C < 1.8, and 75% achieved a ≥ 50% LDL-C reduction as recommended as an alternative in these patients with severe dyslipidemia [4,5,6]. The ESC/EAS also recommend an alternative LDL-C goal of < 1.0 mmol/L to help prevent subsequent CV outcomes in very high-risk patients [5], which almost half of the full cohort achieved, including 56.3% of very high-risk patients with ≥ 2 ASCVD conditions. These impressively low LDL-C levels in very high-risk patients in the real-world setting were associated with a 19–21% reduction in subsequent CV event risk in evolocumab patients with higher baseline CV risk in the FOURIER trial, without increased AEs [28, 29]. Collectively, these findings accentuate CCS recommendations on the patient types expected to derive the greatest benefit from LLT intensification with a PCSK9 inhibitor [6].
While evolocumab is indicated in addition to maximally tolerated background LLT, patients initiated on evolocumab monotherapy achieved a 62.5% LDL-C reduction. This is consistent with a trial in patients with hyperlipidemia and SI wherein evolocumab monotherapy reduced LDL-C by 52.8% after 6 months, which was 36.1% more than that achieved by ezetimibe monotherapy [30]. Hence, the current real-world data are reassuring for the significant SI patient population (61.8% of evolocumab patients in Canada [18]) and 60% in Europe [16] in that international LDL-C guideline recommendations are achievable with evolocumab alone when indicated and clinically appropriate. Still, the difference in LDL-C reductions post evolocumab initiation between the full cohort (75.6% of whom were on a background statin) and patients on monotherapy is consistent with the science showing the impact of PCSK9 inhibition on LDL-C clearance is enhanced when used in combination with a statin [31]. Aligned with current international guidelines [4,5,6], a low background LLT discontinuation rate for patients on evolocumab was observed in the current study, with stable statin use and low rates of ezetimibe discontinuation over 12 months. Hence, when tolerable, the clinical benefit of statin therapy and LLT intensification with a PCSK9 inhibitor and ezetimibe, being the gold standard of care in high- and very high-risk patients with LDL-C above recommended values, appears to be prioritized across the five intercontinental countries studied.
The current findings also add to the growing body of real-world evidence that patients persist on evolocumab, recognizing potential differences between studies in the methodology used [16, 17, 32]. The observed 90.2% evolocumab persistence rate over 12 months is consistent with that reported over 2 years in the European HEYMANS study (92–98%) [16] and in the US GOULD-2 study (92%) [14]. If patients continue to take their medication, as indicated in the current and other real-world studies, the substantial reductions in LDL-C observed over the course of the study are more likely to result in reduced clinical manifestations in routine care [16]. Nevertheless, international real-world evidence shows a lack of persistence to statin therapy, even among most vulnerable patients following an ASCVD event [33,34,35], which may be attributed to intolerance and fear of known side effects [36, 37]. Meanwhile, underlying persistence to evolocumab may be owing to its favourable safety profile, which was observed in the current real-world study to be consistent with that in the evolocumab clinical trial program [21], FOURIER-OLE [25] and other real-world studies [14, 16,17,18]. Notably, only 1 (0.2%) puncture site ecchymosis was reported in the current study, which is lower than the approximately 3–4% of injection site reactions consistently reported in past randomized and OLE trials. This may be reflective of improved patient counselling and administration skills over time. The low incidence of myalgia (0.5%) in the current study is also reassuring, especially considering 35.6% of patients had reported SI, suggesting evolocumab did not exacerbate myalgia.
This retrospective and prospective chart review study is the first intercontinental real-world evaluation of the patient profile initiated on evolocumab, as well as its effectiveness and safety. The study cohort included a representative sample of patients from five countries with varying disease states and indications for PCSK9 inhibition, resulting in a heterogenous population reflective of the real-world dyslipidemia population. A diversity of races was represented with 62.8% of patients being non-White, thus providing data on evolocumab effectiveness and safety in patients who were previously underrepresented in randomized clinical trials [27]. This analysis also included consideration of factors known to impact the effectiveness of evolocumab (e.g. background LLT, persistence), with a robust follow-up of 12 months and 60.9% of patients having ≥ 2 LDL-C measurements post evolocumab initiation. However, important limitations must be addressed, many due to the nature of the chart review study design and the fact data analysis was limited to that collected in the CRF. For one, as the sites selected in the study were anticipated to be specialist sites, they were more used to seeing high/very high CV risk and were more likely to have clinical experience with evolocumab. Hence, our results may be more indicative of clinical experience with evolocumab than is true for sites that do not see as many patients with high/very high CV risk. Furthermore, the possibility of bias resulting from inaccurate chart entry by physicians or staff at the clinical site cannot be ruled out as well. Additionally, the heterogeneity of the population of the five countries and the variability of risk factors on a small sample of patients could be considered a bias. Moreover, though patients were stratified on the basis of CV event risk, an important consideration when understanding the effectiveness of evolocumab in vulnerable patient populations, the CRF did not capture the complete definition of ASCVD according to international guidelines [4,5,6], nor the timing of patients’ past MI or other CV events; hence, some patients may not have been appropriately stratified. Related to this, the limited baseline demographic and clinical data available for analysis may affect the generalizability of the findings. Further, clinical outcomes were not captured as part of this study, which rather focused on the LDL-C response. Still, causal inferences cannot be made, especially considering the frequency of LDL-C monitoring post evolocumab initiation was not structured as in clinical trials. In fact, the COVID-19 pandemic may have affected access and availability of laboratory testing for a small number of patients. Finally, persistence was self-reported by patients, which may limit its validity but the results were compatible with documented persistence of measured LDL-C lowering. Future studies should aim to overcome these data collection limitations to advance understanding of the real-world use and impact of evolocumab on dyslipidemia management in high- and very high-risk patients.
Conclusion
These findings provide insights into evolocumab initiation in routine clinical practice in five countries (Canada, Mexico, Colombia, Saudi Arabia, Kuwait) across three continents, which was demonstrated to be in accordance with its approved indications [21] and international guideline recommendations [4,5,6]. In this heterogeneous population, evolocumab use over 12 months was associated with robust reductions in LDL-C, which were consistent among patients at high and very high CV risk. Overall, the effectiveness and favourable safety profile of evolocumab were similar to randomized clinical trial results [27]. Further, persistence on evolocumab was 90.2%, with relatively stable background LLT. These real-world results signify the potential of evolocumab to help close global dyslipidemia care gaps and reduce modifiable residual CV risk further to improve patient outcomes.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
World Health Organization. Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1. Accessed Aug 26, 2021.
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–97.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–350.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2021;37(8):1129–50.
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-45.
Allen JM, Arnold SV, Lohr NL, et al. Assessing low-density lipoprotein cholesterol risk in secondary prevention patients within the PINNACLE National Outpatient Registry. Circulation. 2019;140:A12904.
Gitt AK, Lautsch D, Ferrières J, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: results from the Dyslipidemia International Study II. Atherosclerosis. 2017;266:158–66.
Al Sifri SN, Almahmeed W, Azar S, et al. Results of the Dyslipidemia International Study (DYSIS)-Middle East: clinical perspective on the prevalence and characteristics of lipid abnormalities in the setting of chronic statin treatment. PLoS One. 2014;9(1): e84350.
Ezetimibe Product Monograph. Sandoz Canada. March 14, 2017. https://www.sandoz.ca/sites/www.sandoz.ca/files/Ezetimibe%20Product%20Monograph.pdf.
Cannon CP, de Lemos JA, Rosenson RS, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6(9):1–9.
Toth PP, Worthy G, Gandra SR, et al. Systematic review and network meta-analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc. 2017;6(10): e005367.
Ray KK, Dhalwani N, Sibartie M, et al. Low-density lipoprotein cholesterol levels exceed the recommended European threshold for PCSK9i initiation: lessons from the HEYMANS study. Eur Heart J Qual Care Clin Outcomes. 2022;8(4):447–60.
Nanchen D, Carballo D, Bilz S, et al. Effectiveness, adherence, and safety of evolocumab in a Swiss multicenter prospective observational study. Adv Ther. 2022;39(1):504–17.
Gupta M, Mancini GBJ, Wani RJ, et al. Real-world insights into evolocumab use in patients with hyperlipidemia: Canadian analysis from the ZERBINI study. CJC Open. 2022;4(6):558–67.
Desai NR, Wade RL, Xiang P, et al. Low-density lipoprotein cholesterol lowering in real-world patients treated with evolocumab. Clin Cardiol. 2021;44(5):715–22.
Al Faraidy K, Akbar M, Shehri M, et al. Multizonal observational study conducted by clinical practitioners on evolocumab use in subjects with hyperlipidemia in Saudi Arabia and Kuwait: results from the ZERBINI study. PLoS One. 2023;18(1):e0278821.
Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10): e1001885.
Repatha (evolocumab) Product Monograph. Amgen Canada Inc. December 9, 2021. https://www.amgen.ca/-/media/Themes/CorporateAffairs/amgen-ca/amgen-ca/documents/products/en/repatha_pm.pdf.
International Diabetes Federation. Diabetes Atlas 10th Edition. Middle East and North Africa. Diabetes Report 2000–2045. https://diabetesatlas.org/data/en/region/4/mena.html. Accessed July 2, 2021.
Schludi B, Giugliano RP, Sabatine MS, et al. Time-averaged low-density lipoprotein cholesterol lowering with evolocumab: pooled analysis of phase 2 trials. J Clin Lipidol. 2022;16(4):538–43.
O’Donoghue ML, Giugliano RP, Wiviott SD, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022;146(15):1109–19.
Qamar A, Giugliano RP, Keech AC, et al. Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab: an analysis of FOURIER trial data. JAMA Cardiol. 2019;4(1):59–63.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Gencer B, Mach F, Murphy SA, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol. 2020;5(8):952–7.
Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from FOURIER. Circulation. 2018;138(8):756–66.
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580–90.
Zhang L, McCabe T, Condra JH, et al. An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes. Int J Biol Sci. 2012;8(3):310–27.
Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol. 2019;74(17):2132–46.
Colantonio LD, Huang L, Monda KL, et al. Adherence to high-intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2(8):890–5.
Guglielmi V, Bellia A, Pecchioli S, et al. Effectiveness of adherence to lipid lowering therapy on LDL-cholesterol in patients with very high cardiovascular risk: a real-world evidence study in primary care. Atherosclerosis. 2017;263:36–41.
Khunti K, Danese MD, Kutikova L, et al. Association of a combined measure of adherence and treatment intensity with cardiovascular outcomes in patients with atherosclerosis or other cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open. 2018;1(8):e185554.
Bates TR, Connaughton VM, Watts GF. Non-adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother. 2009;10(18):2973–85.
Wouters H, Van Dijk L, Geers HC, et al. Understanding statin non-adherence: knowing which perceptions and experiences matter to different patients. PLoS One. 2016;11(1): e0146272.
Toth PP, Descamps O, Genest J, et al. Pooled safety analysis of evolocumab in over 6000 patients from double-blind and open-label extension studies. Circulation. 2017;135(19):1819–31.
Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–82.
Acknowledgements
The authors thank all investigators and patients for their involvement in the ZERBINI Study. The list of investigators can be found in the Supplementary Material online.
Medical Writing and Editorial Assistance
Editorial assistance was provided by Danyelle Liddle, PhD, MSc at MEDUCOM Health Inc. and funded by Amgen Canada Inc.
Funding
This study was funded by Amgen Inc., including design of the study and analysis and interpretation of data. The Rapid Service Fee was funded by Amgen Inc.
Author information
Authors and Affiliations
Contributions
Milan Gupta, Rajvi J. Wani, Khalid Al Faraidy, Jean Bergeron, Eduardo Contreras, Angel Alberto Garcia Peña, G. B. John Mancini, Francisco Padilla, Abel Alberto Pavia Lopez, Kiran Philip, Johnny Wu, and Erin S. Mackinnon contributed to design of the study, review and interpretation of the results, and drafting and review of the manuscript. Milan Gupta, Khalid Al Faraidy, Jean Bergeron, Eduardo Contreras, Angel Alberto Garcia Peña, G. B. John Mancini, Francisco Padilla, and Abel Alberto Pavia Lopez were clinical investigators in the study.
Corresponding author
Ethics declarations
Conflict of Interest
Authors report the following potential conflicts of interest for grants/contracts/consulting fees/honoraria/support for attending meetings or travel/participation on a data safety monitoring board or advisory board. Milan Gupta: Amgen; Khalid Al Faraidy: None declared; Jean Bergeron: Amarin, Amgen, Amryt, ArrowHead, Eli Lilly, HLS Therapeutics, Ionis Pharmaceuticals Inc., Kowa, LIB Therapeutics, Medison, Novartis, Novo Nordisk, Regeneron, Sanofi, The Medicine Co., Ultragenyx; Eduardo Contreras: None declared; Angel Alberto Garcia Peña: Amgen, AstraZeneca, Boehringer, Novartis, Procaps, Pfizer, Sanofi; G. B. John Mancini: Amgen, Esperion, HLS Therapeutics, Novartis, Pfizer, Sanofi; Francisco Padilla: Amgen, Asofarma, AstraZeneca, Ferrer, Servier, Silanes; Abel Alberto Pavia Lopez: Amgen, Ferrer, Novartis, Novo Nordisk, Servier, Stendal, Viatris; Johnny Wu is an employee of Amgen, Inc.; Kiran Philip is an employee of and owns stock in Amgen, Inc.; Rajvi J. Wani and Erin S. Mackinnon are employees of and own stock in Amgen Canada, Inc.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and the study protocol was reviewed and approved by each site’s institutional review board/institutional ethics committee (full list provided in Supplementary Material online). All participants were required to sign an informed consent form for inclusion in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gupta, M., Wani, R.J., Al Faraidy, K. et al. Real-World Insights into Evolocumab Use in Patients with Hyperlipidemia Across Five Countries: Analysis from the ZERBINI Study. Cardiol Ther 12, 703–722 (2023). https://doi.org/10.1007/s40119-023-00334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00334-5