Abstract
Myocarditis is a condition caused by acute or chronic inflammation of the cardiac myocytes, resulting in associated myocardial edema and myocardial injury or necrosis. The exact incidence is unknown, but is likely underestimated, with more mild cases going unreported. Diagnosis and appropriate management are paramount in pediatric myocarditis, as it remains a recognized cause of sudden cardiac death in children and athletes. Myocarditis in children is most often caused by a viral or infectious etiology. In addition, there are now two highly recognized etiologies related to Coronavirus disease of 2019 (COVID-19) infection and the COVID-19 mRNA vaccine. The clinic presentation of children with myocarditis can range from asymptomatic to critically ill. Related to severe acute respiratory syndrome-Coronavirus 2 (SARs-CoV-2), children are at greater risk of developing myocarditis secondary to COVID-19 compared to the mRNA COVID-19 vaccine. Diagnosis of myocarditis typically includes laboratory testing, electrocardiography (ECG), chest X-ray, and additional non-invasive imaging studies with echocardiogram typically being the first-line imaging modality. While the reference standard for diagnosing myocarditis was previously endomyocardial biopsy, with the new revised Lake Louise Criteria, cardiac magnetic resonance (CMR) has emerged as an integral non-invasive imaging tool to assist in the diagnosis. CMR remains critical, as it allows for assessment of ventricular function and tissue characterization, with newer techniques, such as myocardial strain, to help guide management both acutely and long term.
Similar content being viewed by others
Myocarditis results in inflammation of the cardiac myocytes, causing myocardial injury, and remains a recognized cause of sudden cardiac death in children and athletes. |
Myocarditis in children is most often caused by a viral or infectious etiology with two new etiologies related to COVID-19 infection and the COVID-19 mRNA vaccine. |
The clinic presentation of children with myocarditis can range from asymptomatic to critically ill. |
Children are at greater risk of developing myocarditis secondary to COVID-19 compared to the mRNA COVID-19 vaccine. |
While the reference standard for diagnosing myocarditis was previously endomyocardial biopsy, with the new revised Lake Louise Criteria, CMR has emerged as an integral non-invasive imaging tool to assist in the diagnosis. |
Introduction
Myocarditis is a condition caused by acute or chronic inflammation of the cardiac myocyte resulting in associated myocardial edema and myocardial injury or necrosis [1]. The exact incidence is unknown, but is likely underestimated, with more mild cases being asymptomatic or presenting with minimal non-specific symptoms [1, 2]. Several studies have shown a male predominance in those diagnosed with myocarditis, and an estimated incidence of between 0.80 and 2.13 cases per 100,000 (Table 1). Diagnosis and appropriate management are paramount in pediatric myocarditis, as it remains a recognized cause of sudden cardiac death in children and athletes, up to 12% in large studies, mostly based on autopsy [1, 3,4,5,6,7]. In recent years, two new etiologies of myocarditis have emerged, related to severe acute respiratory syndrome-Coronavirus 2 (SARS-CoV-2) and vaccine-associated myocarditis secondary mRNA vaccination. We reviewed and summarized the burden of myocarditis, diagnosis of myocarditis, and newer advanced applications of cardiac magnetic resonance (CMR) assessment of myocarditis in the SARs-CoV-2 era in children. This review is based on previously conducted studies and first-hand experiences and does not include any new studies with human or animal participants.
Pathophysiology
Myocarditis in children is most often caused by a viral or infectious etiology, with a predominance of parvovirus-19 and human herpesvirus 6 [1]. Other less common causes, including autoimmune, medication-related, hypersensitivity reactions, and toxins are also established (Table 2) [1, 7]. From a pathophysiology standpoint, as the pathogen enters the host cell resulting in cell death, an inflammatory cascade of acute inflammatory cells and mediators, such as tumor necrosis factor-α, interleukin-1β, interleukin-6 and nitric oxide, are released. The innate immune response releases neutrophils and monocytes from the bone marrow, the latter of which is thought to drive tissue damage [1]. Several days after infection, the adaptive immune response via antigen-specific T- and B cells clears the virus, but also causes further damage to myocytes, resulting in progression of fibrosis that can lead to the development of cardiomyopathy [1]. After the inflammation subsides, the heart may recover; however, in some instances, persistence of viral presence and inflammation can lead to adverse ventricular remodeling [1, 8]. This can result in evolution to a disease that is indistinguishable from idiopathic dilated cardiomyopathy.
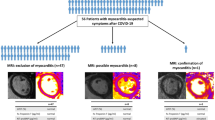
Since 2020, SARS-CoV-2 has emerged as a new, rare infectious cause of myocarditis in children, with and without multisystemic inflammatory syndrome in children (MIS-C) [1, 9,10,11,12]. Early evidence of myocardial involvement with SARS-CoV-2 infection resulted in recommendations for extensive cardiac testing, both in acute SARS-CoV-2 and MIS-C (Fig. 1) [13,14,15,16]. Initial data from collegiate athletes after SARS-CoV-2 infection cited a relatively high prevalence of asymptomatic myocarditis (~ 15%) and caused early alarm [14]. Fortunately, larger follow-up studies have shown a much lower prevalence of less than 1% [17, 18]. Similarly, the initial descriptions of MIS-C showed evidence of significant myocardial injury, with some studies reporting > 90% of patients with troponin elevation. While a significant number of patients present as critically ill, many have a rapid recovery with minimal to no apparent residual sequelae on short-term follow-up [19, 20]. Several studies have noted a distinction between myocarditis caused by SARS-CoV-2 versus the distinct entity of MIS-C, with more global involvement and septal late gadolinium enhancement pattern observed in MIS-C compared to SARS-CoV-2 infection [21]. Patients with myocarditis secondary to MIS-C were also found to have higher C-reactive protein values, variable clinical presentation, and were also noted to have quicker recovery of left ventricular systolic function when compared to other causes of myocarditis [22]. One of the questions regarding this difference is whether the pathophysiology of myocardial injury seen in MIS-C involves a similar process of inflammatory cell infiltration and whether adverse ventricular remodeling can occur. Despite the distinct differences, the etiology of myocarditis in this illness is not well known and long-term sequelae are not yet fully established [21] (Fig. 2).
Evidence of myocarditis versus myocardial involvement after COVID-19. Note abnormalities (denoted by red arrows) in T1 and T2 imaging, which is diagnostic for myocarditis, in the above, compared to only abnormality in T1 imaging without concomitant abnormalities in T2 imaging in the myocardial involvement images
In addition to myocarditis caused by novel virus, vaccine-associated myocarditis has also emerged as one of the newest etiologies. In December 2020, the Food and Drug Administration authorized Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273) for emergency use against SARS-CoV-2. The vaccines were initially authorized for people ≥ 16 years old and ≥ 18 years old, respectively. Both vaccines were administered on a set schedule with the second dose being given 21 days after the first dose for Pfizer and 28 days after the first dose for Moderna [23]. Several cases of myocarditis began to arise after the second dose in older children and adults, now recognized as an adverse event [24]. In large observational studies, SARS-CoV-2 infection continues to have a higher risk of myocarditis (risk ratio of 18.28, CI 3.95–25.12) as compared to the risk with vaccination (risk ratio of 3.24, CI 1.55–12.44), in addition to the other complications that arise from COVID-19 [25]. Despite rare cases of vaccine-associated myocarditis, the Advisory Committee on Immunization Practices has continued to recommend vaccination for all age groups, including adolescents due its benefits outweighing potential risks [23]. More recent data have also suggested that an extended 8-week interval between primary mRNA COVID-19 vaccination series doses may result in a lower risk of myocarditis and pericarditis, as well as improved vaccine effectiveness, which may mitigate some of the major concerns [26].
Clinical Presentation
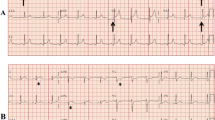
Clinical presentation varies widely, ranging from an asymptomatic to critically ill presentation in children (Fig. 3). In a large pediatric database study, children with myocarditis were more likely to be male with 32% under the age of 4 years old, and 41%, ranging from age 15–18 years old, highlighting a bimodal age at presentation: infancy and adolescence [2, 27]. A higher proportion of sudden death in myocarditis is in males, with myocarditis occurring more frequently in males versus females overall [2, 8]. The most common presenting symptoms include fever and viral prodrome, present in at least 50% of patients, and approximately two-thirds of patients, respectively. Tachypnea and gastrointestinal symptoms, such as nausea with vomiting and abdominal pain, are also common in children [1, 27,28,29,30]. Other common presentations include exercise intolerance, chest pain, dyspnea, and palpitations [27,28,29]. Syncope occurs in 10–12% [1, 27]. More significant presenting symptoms are severe heart failure, ventricular arrhythmias, and sudden death secondary to arrhythmia [27, 31, 32]. As previously noted, the incidence of myocarditis is likely underestimated given the non-specific nature of these symptoms, often resembling more common conditions like asthma and viral gastroenteritis [30]. This wide spectrum of presentation often makes the clinical diagnosis challenging, and typically depends on the specific myocarditis phenotype; the worst being fulminant myocarditis [29, 33, 34]. Of the three main types of myocarditis, acute myocarditis typically presents with systolic ventricular dysfunction either with or without ventricular dilation. Fulminant myocarditis presents with hemodynamic collapse and cardiogenic shock requiring inotropic or mechanical support [1]. In such cases, the left ventricular cavity is of normal size with increased septal thickening (Fig. 2) [35]. Chronic myocarditis is characterized by symptomatic inflammation by laboratory evidence and normal ventricular function. Most cases of myocarditis result in recovery; however, dilated cardiomyopathy and sudden death remain risks in a minority of patients following myocarditis [1]. It is not uncommon for pediatric patients to present with clinical signs/symptoms, evidence of myocardial injury (e.g., troponin leak), but stable hemodynamics and normal left ventricular systolic function. Patients are typically admitted for observation and to monitor for progression, with serial trending of troponins and telemetry monitoring. There are conflicting data regarding the prognostic value of peak troponin, with some data showing higher peak troponin correlating with worse outcome [36]. While worse outcomes with ventricular dysfunction at presentation are well established, patients with normal left ventricular systolic function at presentation are also at risk for evolving dysfunction necessitating treatment [37].
Example of fulminant myocarditis. Note the extensive lateral wall thickening seen during the acute episode that has resolved back to normal (yellow arrow), consistent with myocardial edema. This area also showed extensive delayed myocardial enhancement on MRI that has also resolved (green arrows). There is an additional pericardial effusion seen (red arrow), consistent with perimyocarditis
Vaccine-associated myocarditis has been reported more commonly in adolescent and adult males and recovery has typically been swift and complete in most cases [38, 39]. In a recent single institution study, vaccine-associated myocarditis was associated with less biomarker inflammation, specifically brain natriuretic peptide (BNP) and troponin compared to classic myocarditis and MIS-C myocarditis; however, electrocardiogram (ECG) changes were seen in 67% of patients. Pediatric patients with vaccine-associated myocarditis had a shorter median length of stay (3 days) compared to previously reported multi-institutional data of non-SARS-CoV-2 myocarditis [2]. All vaccine-associated myocarditis patients had normal function at the time of discharge, compared to classic myocarditis and MIS-C myocarditis [40].
Evaluation
When myocarditis is suspected, patients will typically undergo laboratory testing, ECG, chest X-ray, and additional non-invasive imaging studies (such as echocardiography, cardiac magnetic resonance (CMR), or cardiac computed tomography). Specific testing and biomarkers may be diagnostic; however, these findings may not be present in pediatric patients with myocarditis, which can make the diagnosis challenging. Initial assessment includes cardiac specific biomarkers, such as troponin level, creatinine kinase MB, BNP/pro-BNP, some of which have been associated with poor outcome [1, 41]. Other nonspecific lab markers of inflammation may also be useful, although are not always obtained [1]. ECG may show numerous abnormalities in children; however, wide QRS-T angle, low voltage, and prolonged QTc have been associated with major adverse cardiac events (MACE) [1, 42]. Of all types of myocarditis, ECG changes are least common in MIS-C myocarditis [40].
Endomyocardial Biopsy
Endomyocardial biopsy remains the reference standard for diagnosis of myocarditis. The Dallas criteria, initially published in 1986, served as the first histopathological criteria for myocarditis establishing the presence of inflammatory cellular infiltrate with or without necrosis as criteria for diagnosis (Fig. 4) [1, 43, 44]. Recent advancements, such as advanced immunohistochemistry techniques, have improved the sensitivity of detecting inflammatory cells infiltrates. Expression of human leukocyte antigen (HLA) and inflammatory markers can support a diagnosis of myocarditis, where basic histology is inconclusive [1]. However, while endomyocardial biopsy (EMB) with immunohistology is considered the invasive reference standard for diagnosis of myocarditis, biopsy is overall declining in children given the emergence of other safe modalities to diagnose myocarditis due to a variety of reasons [1].
Biopsy samples are obtained most commonly from the right ventricular side of the interventricular septum, although EMB from the right ventricular lateral wall or left ventricle can be performed in rare instances. A bioptome is passed into the heart and multiple samples are typically obtained, although the risk for complications may increase with additional samples. One of the major limitations of EMB is sampling error, which reduces its sensitivity [45]. Several autopsy and CMR-based studies have shown that the earliest myocardial abnormalities in acute myocarditis are typically seen in the lateral wall of the left ventricle, whereas EMB is most commonly performed in the right ventricle [46]. An autopsy-based study by Hauck et al. found that out of 38 heart specimens with lymphocytic myocarditis with ten post-mortem endomyocardial specimens taken from the right ventricular apical septum, only 63% were positive for histological myocarditis [47]. There is also significant variation in the interpretation of biopsy specimens, even among expert pathologists [48]. The invasive nature of EMB is another reason for its decline in utilization. A 6% complication rate is reported in the literature, including bleeding, arrhythmia, and perforation causing pericardial tamponade. These complications are influenced by characteristics of the patient, experience of the provider, and conditions under which the biopsy is performed [49]. When looking specifically at children undergoing EMB, a higher complication rate has been cited in the literature, with younger age, smaller size, and need for inotropic support shown to be risk factors [50, 51].
While utilization is declining, there are instances where EMB is recommended. Class I indications for EMB are new-onset heart failure less than 2 weeks with hemodynamic compromise irrespective of left ventricular size, new-onset heart failure 2 weeks to 3 months in duration associated with left ventricle dilation, ventricular arrhythmias, atrioventricular block, and failure of response to treatment in 1–2 weeks [49]. However, many of these indications are mainly applicable to adults. The reasons for biopsy within pediatrics remain debated. Thus, in the recent era, CMR use in the diagnosis of myocarditis in pediatric patients continues to increase, and has increased up to 37%, more often in the acute hospital setting, with subsequent decline in catheterization in those who underwent CMR during the same period [52].
Echocardiography
Transthoracic echocardiography (TTE) is the first-line assessment of ventricular systolic function in pediatric patients due to its ability to make real-time images, its portability, and its ability to provide useful diagnostic information relatively quickly [1]. It also enables cardiologists to evaluate for cardiac findings that necessitate continual assessment, such as diminished ventricular function, valvar regurgitation, pericardial effusion, and intracardiac thrombus. Worse ventricular function, including depressed left ventricular ejection fraction (LVEF), has been associated with poor outcomes and increased risk of death or transplantation [28, 53]. Some novel techniques continue to be developed to help in the diagnosis of evaluation of myocarditis. Myocardial strain is an advanced imaging technique that evaluates subclinical regional wall motional abnormalities that can also be obtained with echocardiography. There are some data demonstrating worse global longitudinal strain in patients with CMR evidence of myocarditis in the absence of ventricular function abnormalities in both single-center and multicenter studies and may serve as an additional non-invasive marker of disease [1, 54]. Despite these recent advances, TTE continues to play a more important role in surveillance and clinical management and is less reliable in the diagnosis of myocarditis. While increased wall-thickness, as seen in Fig. 3, with concomitant regional wall motion abnormalities can be suggestive of the diagnosis, many of the common TTE findings in myocarditis are non-specific. Cases with significantly increased wall thickness can meet the diagnostic criteria for hypertrophic cardiomyopathy, while severely dilated and dysfunctional left ventricles can be confused for dilated cardiomyopathy [55]. A three-tier diagnostic algorithm has been developed for myocarditis utilizing symptoms and diagnostic tools, with recent adaptations for pediatric myocarditis given the rapidly increasing utilization of CMR. Specifically, CMR is now characterized as confirmatory, instead of suspected in the diagnosis of myocarditis [1, 35].
Cardiac Magnetic Resonance Imaging
CMR has emerged as an integral non-invasive imaging tool with good sensitivity and specificity, decreasing the use of EMB in the diagnosis of myocarditis. The advantages of CMR are its non-invasive nature, ability to assess the entire myocardium, and its ability to obtain additional structural and hemodynamic information. The disadvantages involve the need for breath-holding, which can require sedation in younger children, difficulty in very small children with signal–noise limitations and high heart rates, and the need for intravenous gadolinium-based contrast [56]. A CMR may also be technically infeasible in critically unstable patients or in those requiring mechanical circulatory support. However the risk–benefit in the majority of pediatric patients is weighted towards CMR, with practice patterns reflecting this. O’Halloran et al. demonstrated this by utilizing the Pediatric Health Information System (PHIS) to show the increasing utilization of CMR and concomitant decreasing utilization of EMB over a 15-year period [52].
For CMR, the Lake Louise Criteria (LLC) are used to diagnose acute myocarditis, due to its ability to demonstrate abnormal markers of inflammation and necrosis (Fig. 5) [1, 45]. Based on original LLC from 2009, myocarditis by CMR is diagnostic when two of three findings are present: regional or global myocardial signal intensity increase in T2-weighted images, increased global myocardial early gadolinium enhancement ratio between skeletal muscle in T1-weighted images, and at least one focal lesion in non-ischemic distribution in late gadolinium enhancement (LGE) imaging [45, 57]. The revised Lake Louise Criteria (rLLC) in 2018 now include: one T1-based criterion (prolonged T1 relaxation time, elevated extracellular volume fraction or the presence of LGE), and at least one T2-based criterion (increased T2 relaxation time, increased signal intensity in areas of edema, and increased T2 signal ratio of myocardium to skeletal muscle) [57, 58]. With the addition of quantitative parametric mapping techniques, CMR has become more sensitive (87.5%) in diagnosing myocarditis with important prognostic and diagnostic implications [57, 58]. The addition of parametric mapping allows for more objective assessment of abnormality, though some important caveats should be considered. Normative data should be obtained, and is specific to the scanner and acquisition sequence used. The amount of time from contrast administration is also important for post-contrast T1 mapping and should remain consistent for all scans [27]. More specifically for pediatrics, elevated heart rates can introduce variability, although different sequences or variations in pulse sequences can mitigate this [59].
Revised Lake Louise Criteria for cardiac MRI diagnosis of myocarditis. The patient must have abnormalities in any T1 sequence (representing non-ischemic myocardial injury) and in any T2 sequence (representing myocardial edema). Supporting criteria are evidence of pericarditis (pericardial effusion, or abnormalities in the pericardium) and/or evidence of systolic left ventricular dysfunction (regional or global wall motion abnormalities)
Parametric mapping differs in the acute phase of myocarditis compared to follow-up assessment. Prolonged T1 relaxation time is highly sensitive for myocarditis [58, 60]. A study by Luetkens et al. found that during the acute phase, the majority of patients were found to have an elevated T2 ratio, early gadolinium enhancement, prolonged native T1 relaxation time, prolonged T2 relaxation times, and elevated ECV fraction. All markers subsequently decreased over time, although myocardial edema (as measured by T2 mapping) showed an acute decrease and normalized by 3 weeks after diagnosis [61]. T2 mapping showed the best ability to discriminate between acute and healed stages of myocarditis, as these values normalized with time compared to other mapping parameters [27, 61, 62]. In a large multi-center pediatric cohort, positive T2 and LGE were significantly more common in patients who did not fully recovery from myocarditis [63]. When risk stratifying myocardial mapping to primary endpoint, native T1 mapping and ECV fraction have been significantly associated with MACE and death; specifically, an ECV fraction greater ≥ 35% [64, 65]. One study also demonstrated an association between abnormal T2 ratio and poor outcome [64].
Late gadolinium enhancement is the most extensively studied prognostic indicator of poor outcome in patients with myocarditis. In areas of inflamed myocardium, intravenous contrast clearance in the extracellular space is delayed compared to healthy myocardium, resulting in enhancement on T1-weighted imaging. Late gadolinium enhancement is described by location and characterized by its appearance; often described as anteroseptal versus posterolateral and patchy versus epicardial [46]. In a small retrospective analysis, LGE was found to be associated with early and late poor outcomes, specifically extracorporeal membrane oxygenation, ventricular assist device, and death [41]. Failure of recovery, ventricular arrhythmia, and death have been associated with mid-wall distribution and anteroseptal location in both children and adults compared to posterolateral distribution [31, 32, 64, 66,67,68,69]. In multivariate analysis, LGE is associated with poor outcome, in addition to severely decreased left ventricular systolic function [41]. A recent multicenter study by Ait-Ali et al. demonstrated the prognostic value of LGE in pediatrics as well, showing that midwall, full-thickness, and patchy distributions of LGE were less likely to recover ventricular size and function on follow-up imaging at a median time of 5 months [63, 68].
Myocardial strain, which measures the change in muscle fiber length overtime, has emerged as a useful adjunctive tool to assess ventricular function due to is ability to predict occult dysfunction in patients with myocarditis and preserved left ventricular ejection fraction. Multiple strain techniques including CMR feature tracking (CMR-FT), myocardial tagged imaging, and strain encoded imaging have been studied in small cohorts. Reduced strain by CMR-FT is also associated with MACE. In multivariate analysis, GLS was independently associated with MACE, which supports the susceptibility of longitudinally orientated myocardial fibers to dysfunction [67]. However, the diagnostic and prognostic capabilities of strain by CMR continue to be studied.
Clinical Treatment and Follow-up
The treatment of myocarditis varies depending on the severity of presentation and stage of illness. Both atrial and ventricular arrhythmias are of consideration and require appropriate management with antiarrhythmics, as ventricular arrhythmias are associated with poor outcome. Presentation with complete heart block has been reported but is rare. Temporary pacing is an additional mechanism to treat patients with dysrhythmia. Continuous cardiac monitoring as an inpatient is important for patients with myocarditis, especially in patients with ventricular dysfunction [1].
For decompensated patients, inotropic support is typically initiated with milrinone, a phosphodiesterase-3 inhibitor, which improves ventricular contractility, afterload reduction, and improved relaxation or lusitropy. Inotropic agents with vasopressor activity, such as epinephrine, are reserved for hypotension and cardiogenic shock [1]. Calcium chloride and vasopressin can also be used to augment systemic perfusion. In cases of fulminant myocarditis, or those refractory to medical treatment, mechanical circulatory support (MCS) at an appropriate center should be considered early in patients with cardiogenic collapse. Pediatric registry data show that up to 23% of patients may need treatment with MCS during hospitalization. Extracorporeal membrane oxygenation (ECMO) has the unique ability to be deployed emergently as a short-term lifesaving measure. Multicenter data suggest that weaning from ECMO is favorable in the majority of patients who present with cardiogenic shock and is associated with improved survival [1]. In patients who require long-term support, ventricular assist devices (VADs), such as the Berlin Heart EXCOR, are a more chronic form of support for patients who will need a bridge to transplantation [1, 35]. Newer support devices, such as Impella (Abiomed, Danvers, MA, USA), offer benefit in left ventricular unloading through less invasive percutaneous placement [1, 70].
For more stable patients, management typically consists of an oral heart failure regimen, such as diuretic therapy to decrease venous congestion, angiotensin-converting enzyme inhibitor (ACE-I) and angiotensin II receptor blockers for afterload reduction, and beta-blockers. Aldosterone antagonists are also used for ventricular remodeling. The most studied beta-blocker, carvedilol, has been shown to be cardioprotective in animal studies and supports ventricular remodeling [35]. This practice is seen across many pediatric centers. A retrospective multicenter study of pediatric patients hospitalized with myocarditis found that 64% of transplant-free survival patients were discharged on heart failure medications; the most common being an ACE-I, followed by a beta-blocker [28].
Immunosuppressive therapy with non-steroidal anti-inflammatory drugs has been shown to be beneficial for patients with concomitant pericarditis and pericardial effusion; however, clinical improvement is difficult to extrapolate to myocarditis [35]. Despite mixed results in mostly adult trials of steroid use, immunosuppression with steroids is still a common treatment in patients with myocarditis. A large Cochrane review of multiple randomized controlled trials regarding the use of steroids determined that there was no difference in mortality if steroids were used in children or adults; however, left ventricular ejection fraction was higher if steroids were used. The strength of the data was limited by extensive heterogeneity in the studies, and only two studies included were pediatric-focused [71]. Immunologic therapy with intravenous immunoglobulin (IVIG) has also been utilized in the treatment of myocarditis. IVIG has shown to improve left ventricular systolic function [35]. A recent retrospective analysis showed that the combination of IVIG and high-dose steroids was beneficial for improving left ventricular systolic function without significant serious adverse events [54]. In a national retrospective database study, the pediatric IVIG cohort experienced fewer deaths than the pediatric cohort treated with steroids [72]. Despite mixed data, pediatric centers still use both IVIG and steroids to treat myocarditis. One center reported 92.5% transplant-free survival over 1 year with improvement in echocardiographic indices [54]. With the emergence of SARS-CoV-2 and MIS-C, many pediatric centers have introduced newer treatment algorithms that utilize immunoglobin, corticosteroids, and aspirin to improve ventricular function and prevent coronary aneurysm [1, 40, 73]. A large survey of many pediatric centers showed that IVIG and steroids were used in 99% and 93% of MIS-C protocols, respectively. Their use increased as the severity of illness worsened [73]. Other classes of medications are less well studied in pediatrics. Newer treatments include interferon α and β, which have shown some benefit in left ventricular function in small, single-center studies[35]. There is weak evidence to definitively support treatment with antiviral medication in pediatric patients; however, treatment is reasonable in the case of active infection where involvement of the myocardium is not known. Rare forms of non-infectious etiologies of myocarditis, such as giant cell myocarditis and eosinophilic myocarditis, are known to respond well to immunosuppression with steroids [1].
Factors that are associated with a poor outcome include limited functional status, elevated BNP, elevated troponin, tachyarrhythmias, and ventricular dysfunction [74]. There is new evidence to suggest that recovery of ventricular function differs based upon the etiology of myocarditis in the short term. In a single-center retrospective study of patients hospitalized with myocarditis, most patients saw recovery in function; however, only 47% of patients with classic myocarditis have recovery of function, compared 76% of patients with MIS-C myocarditis and 100% of patients with vaccine-associated myocarditis [40]. A single-center study showed that most patients who presented with myocarditis had recovery of ventricular function, up to 60 months out from initial presentation [54]. Despite recovery of ventricular function, myopathy can still occur later in life [1]. Long-term management and follow-up strategies are limited in pediatrics.
Management of myocarditis in athletes is of particular importance due to significant risk with strenuous exercise in this patient population. Once the diagnosis is made, surveillance and restriction from competitive sports help to mitigate risks of sudden death [75]. The American Heart Association guidelines recommend repeat assessment with a resting echocardiogram, 24-Holter monitoring, and exercise ECG no less than 3–6 months following acute illness [75]. Athletes can return to play with normalization of myocardial inflammation or injury, normalization of left ventricular systolic function, and absence of arrhythmia on Holter assessment [75].
While most edema and LGE resolve, persistent changes of depressed ventricular function, scar, and ventricular arrhythmias can occur [76]. In a small adult cohort, LGE persisted or increased in the majority of patients. Twenty percent of patients with increased LGE had a MACE reported during the study period [77]. Years after resolution of initial insult, myopathy can result as a result of abnormal ventricular remodeling. In a large Pediatric Cardiomyopathy Registry study, patients who presented with reduced systolic function and had a left ventricular end-diastolic dimension z-score greater than 2 saw lower rates of normalization. At 3 years following initial presentation, 54% of biopsy-proven myocarditis and 52% of probable myocarditis patients had normalization of function. In multivariate analysis, left ventricular fractional shortening was predictive of death, and left ventricular posterior wall dimension z-score predicted transplantation. Seventy-two percent of children with a normal fractional shortening without dilation saw normalization by 3 years compared to 46% of patients with dilation [78].
Conclusions
Myocarditis is a rare inflammatory condition in children, which requires timely diagnosis and appropriate management for prognostication and risk stratification. SARS-CoV-2 is the newest infectious cause of myocarditis, with which long-term sequelae are unknown. Cardiac MRI is an important non-invasive tool that allows for assessment of ventricular function and tissue characterization, with newer techniques, such as myocardial strain, to help guide management acutely and for the long term. Despite resolution of myocardial injury and inflammation, long-term multimodality reassessment is beneficial due to the potential development of heart failure and MACE.
Data availability
No datasets were analyzed or generated during the course of this review article.
References
Law YM, Lal AK, Chen S, Cihakova D, Cooper LT Jr, Deshpande S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144(6):e123–35.
Vasudeva R, Bhatt P, Lilje C, Desai P, Amponsah J, Umscheid J, et al. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007–2016. Am J Cardiol. 2021;149:95–102.
Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119(8):1085–92.
Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92(3):316–20.
Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180(3):110–2.
Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm. 2005;2(12):1277–82.
Harris KM, Mackey-Bojack S, Bennett M, Nwaudo D, Duncanson E, Maron BJ. Sudden unexpected death due to myocarditis in young people including athletes. Am J Cardiol. 2021;143:131–4.
Levine BDB, Kovacs AL, Link RJ, Maron MS, Mitchell MS, Jere H. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 1: classification of sports: dynamic, static, and impact: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;66:2350–5.
Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–36.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334–46.
Wu EY, Campbell MJ. Cardiac manifestations of multisystem inflammatory syndrome in children (MIS-C) following COVID-19. Curr Cardiol Rep. 2021;23(11):168.
Fick TA, Cua CL, Lee S. Imaging findings in pediatric COVID-19: a review of current literature. Cardiol Ther. 2022;11(2):185–201.
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–73.
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020;6:116–8.
Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143(6):609–12.
Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32.
Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;9:1078–87.
Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–52.
Farooqi KM, Chan A, Weller RJ, Mi J, Jiang P, Abrahams E, et al. Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. 2021;148:e2021051155.
Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolescent Health. 2021;5(7):473–82.
Li DL, Davogustto G, Soslow JH, Wassenaar JW, Parikh AP, Chew JD, et al. Characteristics of COVID-19 myocarditis with and without multisystem inflammatory syndrome. Am J Cardiol. 2022;168:135–41.
Vukomanovic VA, Krasic S, Prijic S, Ninic S, Minic P, Petrovic G, et al. Differences between pediatric acute myocarditis related and unrelated to SARS-CoV-2. Pediatr Infect Dis J. 2021;40(5):e173–8.
Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–82.
Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219–27.
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–9.
Wallace M, Moulia D, Blain AE, Ricketts EK, Minhaj FS, Link-Gelles R, et al. The advisory committee on immunization practices’ recommendation for use of Moderna COVID-19 vaccine in adults aged >/=18 years and considerations for extended intervals for administration of primary series doses of mRNA COVID-19 vaccines—United States, February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):416–21.
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19(1):75.
Butts RJ, Boyle GJ, Deshpande SR, Gambetta K, Knecht KR, Prada-Ruiz CA, et al. Characteristics of clinically diagnosed pediatric myocarditis in a contemporary multi-center cohort. Pediatr Cardiol. 2017;38(6):1175–82.
Durani Y, Egan M, Baffa J, Selbst SM, Nager AL. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009;27(8):942–7.
Freedman SB, Haladyn JK, Floh A, Kirsh JA, Taylor G, Thull-Freedman J. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics. 2007;120(6):1278–85.
Ali-Ahmed F, Dalgaard F, Al-Khatib SM. Sudden cardiac death in patients with myocarditis: evaluation, risk stratification, and management. Am Heart J. 2020;220:29–40.
Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, De Cobelli F, et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75(9):1046–57.
Amabile N, Fraisse A, Bouvenot J, Chetaille P, Ovaert C. Outcome of acute fulminant myocarditis in children. Heart. 2006;92(9):1269–73.
Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. 2012;76(5):1222–8.
Dasgupta S, Iannucci G, Mao C, Clabby M, Oster ME. Myocarditis in the pediatric population: a review. Congenit Heart Dis. 2019;14(5):868–77.
Butto A, Rossano JW, Nandi D, Ravishankar C, Lin KY, O’Connor MJ, et al. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: an analysis of the PHIS+ database. Pediatr Cardiol. 2018;39(6):1139–43.
Barfuss SB, Butts R, Knecht KR, Prada-Ruiz A, Lal AK. Outcomes of myocarditis in patients with normal left ventricular systolic function on admission. Pediatr Cardiol. 2019;40(6):1171–4.
Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SA, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5):e2021053427.
Dionne A, Son MBF, Randolph AG. An update on multisystem inflammatory syndrome in children related to SARS-CoV-2. Pediatr Infect Dis J. 2022;41(1):e6–9.
Patel T, Kelleman M, West Z, Peter A, Dove M, Butto A, et al. Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children. J Am Heart Assoc. 2022;11(9): e024393.
Sachdeva S, Song X, Dham N, Heath DM, DeBiasi RL. Analysis of clinical parameters and cardiac magnetic resonance imaging as predictors of outcome in pediatric myocarditis. Am J Cardiol. 2015;115(4):499–504.
Fischer K, Marggraf M, Stark AW, Kaneko K, Aghayev A, Guensch DP, et al. Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS ONE. 2020;15(1): e0227134.
Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113(6):876–90.
Ht A, Me B, Wd E, Sm F, Jt F Jr, FJ, et al. Myocarditis: a histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3–14.
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475–87.
Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–8.
Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235–45.
Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, et al. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75(2):401–5.
Cooper LT. Myocarditis. N Engl J Med. 2009;360(15):1526–38.
Pophal SG, Sigfusson G, Booth KL, Bacanu SA, Webber SA, Ettedgui JA, et al. Complications of endomyocardial biopsy in children. J Am Coll Cardiol. 1999;34(7):2105–10.
Pilati M, Rebonato M, Formigari R, Butera G. Endomyocardial biopsy in pediatric myocarditis and dilated cardiomyopathy: a tool in search for a role. J Cardiovasc Dev Dis. 2022;9(1):24.
O’Halloran CP, Robinson JD, Watanabe K, Zumpf KB, Petito LC, Marino BS, et al. Magnetic resonance imaging in pediatric myocarditis: trends and associations with cost and outcome. JACC Cardiovasc Imaging. 2022;15(7):1230–8.
Magnani JW, Danik HJ, Dec GW Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151(2):463–70.
Schauer J, Newland D, Hong B, Albers E, Friedland-Little J, Kemna M, et al. Treating pediatric myocarditis with high dose steroids and immunoglobulin. Pediatr Cardiol. 2022;44:1–10.
Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48(10):2085–93.
Choi JW, Moon WJ. Gadolinium deposition in the brain: current updates. Korean J Radiol. 2019;20(1):134–47.
Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, et al. Comparison of original and 2018 Lake Louise Criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging. 2019;1(3):e190010.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–76.
Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16(1):2.
Blissett S, Chocron Y, Kovacina B, Afilalo J. Diagnostic and prognostic value of cardiac magnetic resonance in acute myocarditis: a systematic review and meta-analysis. Int J Cardiovasc Imaging. 2019;35(12):2221–9.
Luetkens JA, Homsi R, Dabir D, Kuetting DL, Marx C, Doerner J, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc. 2016;5(7):e003603.
von Knobelsdorff-Brenkenhoff F, Schuler J, Doganguzel S, Dieringer MA, Rudolph A, Greiser A, et al. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2017;10(2):e005242.
Ait-Ali L, Martins DS, Khraiche D, Festa P, Barison A, Martini N, et al. Cardiac MRI prediction of recovery in children with acute myocarditis. JACC Cardiovasc Imaging. 2021;14(3):693–5.
Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–76.
Grani C, Biere L, Eichhorn C, Kaneko K, Agarwal V, Aghayev A, et al. Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. Int J Cardiovasc Imaging. 2019;35(6):1067–78.
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70(16):1977–87.
Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, et al. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. JACC Cardiovasc Imaging. 2020;13(9):1891–901.
Martins DS, Ait-Ali L, Khraiche D, Festa P, Barison A, Martini N, et al. Evolution of acute myocarditis in a pediatric population: An MRI based study. Int J Cardiol. 2021;329:226–33.
Eichhorn C, Greulich S, Bucciarelli-Ducci C, Sznitman R, Kwong RY, Gräni C. Multiparametric cardiovascular magnetic resonance approach in diagnosing, monitoring, and prognostication of myocarditis. JACC Cardiovasc Imaging. 2022;15:1325–38.
Dimas VV, Morray BH, Kim DW, Almond CS, Shahanavaz S, Tume SC, et al. A multicenter study of the Impella device for mechanical support of the systemic circulation in pediatric and adolescent patients. Catheter Cardiovasc Interv. 2017;90(1):124–9.
Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013;2013(10):CD004471.
Lin MS, Tseng YH, Chen MY, Chung CM, Tsai MH, Wang PC, et al. In-hospital and post-discharge outcomes of pediatric acute myocarditis underwent after high-dose steroid or intravenous immunoglobulin therapy. BMC Cardiovasc Disord. 2019;19(1):10.
Dove ML, Jaggi P, Kelleman M, Abuali M, Ang JY, Ballan W, et al. Multisystem inflammatory syndrome in children: survey of protocols for early hospital evaluation and management. J Pediatr. 2021;229:33–40.
Putschoegl A, Auerbach S. Diagnosis, evaluation, and treatment of myocarditis in children. Pediatr Clin N Am. 2020;67(5):855–74.
Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273–80.
Małek ŁA, Kamińska H, Barczuk-Falęcka M, Ferreira VM, Wójcicka J, Brzewski M, et al. Children with acute myocarditis often have persistent subclinical changes as revealed by cardiac magnetic resonance. J Magn Reson Imaging. 2020;52(2):488–96.
Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ Heart Fail. 2017;10(11):e004262.
Foerster SR, Canter CE, Cinar A, Sleeper LA, Webber SA, Pahl E, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3(6):689–97.
Arola A, Pikkarainen E, Sipila JO, Pykari J, Rautava P, Kyto V. Occurrence and features of childhood myocarditis: a nationwide study in Finland. J Am Heart Assoc. 2017;6(11):e005306.
Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, Slonim AD. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatr Cardiol. 2010;31(2):222–8.
Acknowledgements
We thank Dr. Peter Baker for providing images of pathology specimens.
Funding
No funding or sponsorship was received for the publication of this article.
Author Contributions
Dr. Jason L. Williams and Dr. Simon Lee created the concept and design of the manuscript. Dr. Jason Williams, Dr. Simon Lee, and Dr. Hannah M. Jacobs drafted and edited the manuscript and performed revisions.
Disclosures
Jason L. Williams, Hannah M. Jacobs, and Simon Lee have nothing to disclose.
Compliance with Ethics Guidelines
This review is based on previously conducted studies and first-hand experiences and does not include any new studies with human or animal participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, J.L., Jacobs, H.M. & Lee, S. Pediatric Myocarditis. Cardiol Ther 12, 243–260 (2023). https://doi.org/10.1007/s40119-023-00309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00309-6